Barbieri Laboratory Research Areas
My laboratory studies the molecular basis of microbial pathogenesis in Cellular Microbiology where components of the pathogen and host are analyzed to understand the pathogenic process. My research involves the study of bacterial toxins. Several families of bacterial toxins are under investigation: botulinum and tetanus neurotoxins; Certhrax, an ADP-ribosylating exotoxin from Bacillus cereus; and ExoS, a type III cytotoxin of Pseudomonas aeruginosa.
Botulinum toxin and Tetanus toxin
In 2003, I joined the Great Lakes Regional Center of Excellence (GLRCE) and began studies on the botulinum neurotoxins (BoNTs) and tetanus toxin. These neurotoxins have dual specificity for neurons, binding neuron-specific host receptors and cleaving neuron-specific substrates, SNARE proteins. The central theme is to understand the molecular basis for the flaccid paralysis elicited by BoNTs relative to the spastic paralysis elicited by Tent. We defined the basis for SNARE substrate specificity and licensed a BoNT LC-variant, which cleaved a non-neuronal SNARE protein, which may extend therapeutic potential beyond neurological diseases. We identified the dual receptors for several BoNT serotypes and determined the host receptors of tetanus toxin to be dual gangliosides.
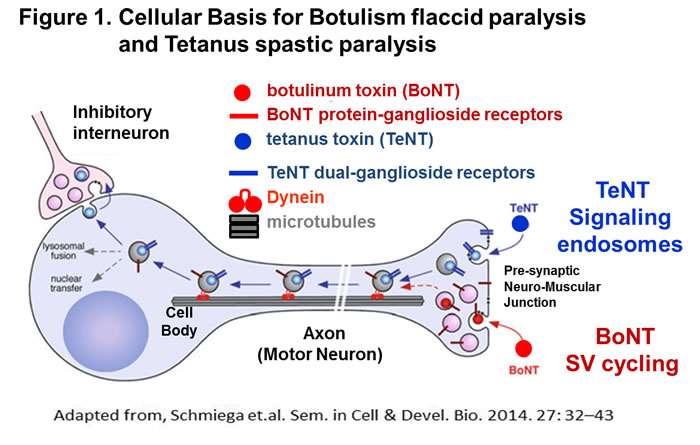
We engineered the first recombinant subunit vaccine against the seven serotypes of BoNTs and recently developed the first protein platform to deliver therapies that neutralized intracellular BoNT in neurons. We utilize two model cell systems to study the entry tetanus toxin (TeNT) into neurons, primary neurons and cultured neuronal cell lines. These studies showed the unique entry of TeNT into signaling endosomes independent of synaptic vesicle cycling. We determined that entry into signaling endosomes was mediated by regions of TeNT that lied outside the receptor-binding domain (HCR). In addition, we recently developed an assay to measure LC translocation into the cytosol of neurons, the least understood step of toxin action. Over the past ~10 years, my laboratory has also conducted basic research to define how BoNTs enter neurons and translational studies to develop vaccines and therapies against botulism. Representative publications:
- A heterologous reporter defines the role of the tetanus toxin inter-chain disulfide in Light Chain translocation
- Multiple domains of tetanus toxin direct entry into primary neurons
- Enhancing the protective immune response against botulism
- Tetanus toxin and botulinum toxin A utilize unique mechanisms to enter neurons of the central nervous system
- Engineering botulinum neurotoxin to extend therapeutic intervention
Bacillus cereus Certhrax
In 2012, I began a collaboration with Alison O’Brien, PhD (Uniform Services University) to characterize Certhrax, a predicted ADP-ribosyltransferase from Bacillus cereus. While B. cereus is often associated with mild to moderate gastroenteritis, some recent isolates cause inhalational anthrax-like disease. These potential emerging human pathogens express multiple virulence factors where B. cereus strain G9241 expresses anthrax toxin, several polysaccharide capsules, and Certhrax. We have shown that Certhrax ADP-ribosylates R433 on vinculin, a host scaffolding protein that coordinates actin cytoskeleton and extracellular matrix interactions.
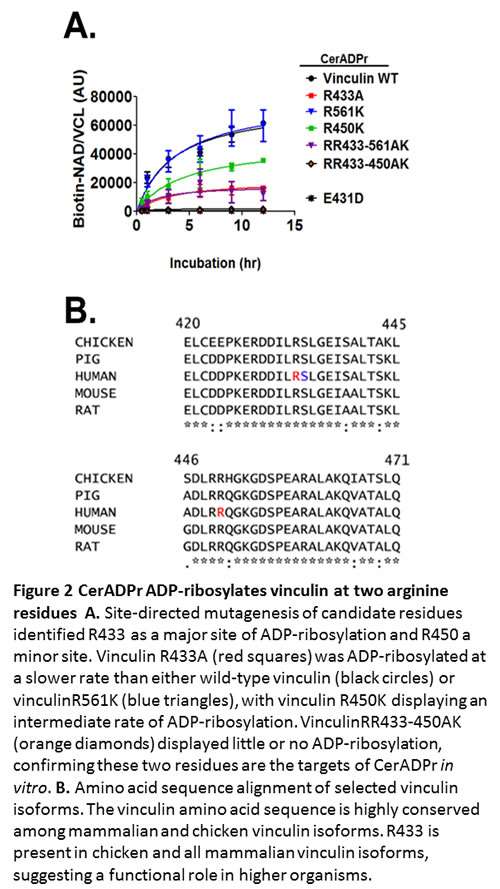
ADP-ribosylation of vinculin disrupts focal adhesion complexes and redistributes vinculin to the cytoplasm. This provides a mechanism for strain G9241 to breach host barrier defenses and promote bacterial growth and spread. Certhrax is the first bacterial toxin to add a post-translational modification to vinculin to disrupt the actin cytoskeleton. We continue to collaborate with Dr. O’Brien to understand how Certhrax contributes to the molecular and cellular pathogenesis of B. cereus. Representative publications:
- Bacillus cereus Certhrax ADP-ribosylates vinculin to disrupt focal adhesion complexes and cell adhesion
- Novel bacterial ADP-ribosylating toxins: structure and function
- Host cell cytotoxicity and cytoskeleton disruption by CerADPr, an ADP-ribosyltransferase of Bacillus cereus G9241
Pseudomonas aeruginosa ExoS and ExoT
I collaborated with Dara Frank, PhD (MCW) to determine the molecular and cellular properties of ExoS and ExoT, type III cytotoxins of P. aeruginosa. We cloned the genes encoding exoS and exoT and then determined how ExoS ADP-ribosylated Ras and how ExoT ADP-ribosylated Crk to uncouple host signal transduction. We determined that ExoS and ExoT had dual catalytic domains, encoding both an ADP-ribosyltransferase domain and Rho-GTPase activating (Rho-GAP) domain and showed that ExoS utilized vesicle-associated, intracellular trafficking to ADP-ribosylate Ras.
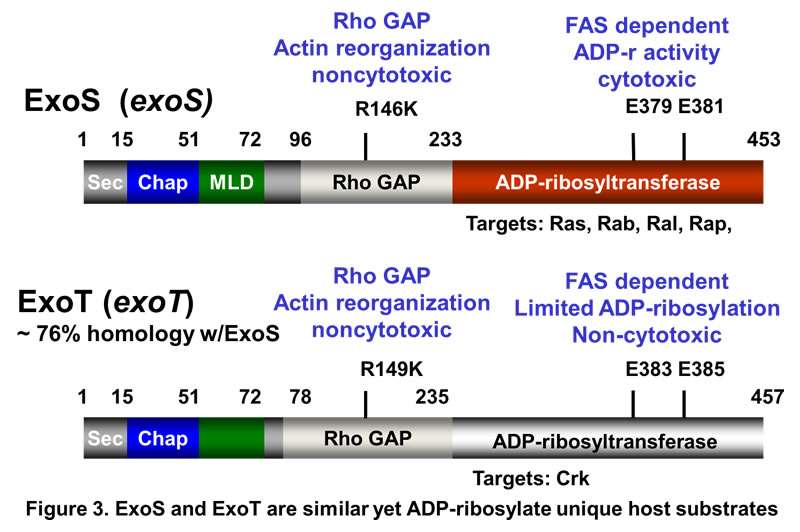
We continue to study the cellular microbiology of ExoS in collaborations with Suzie Fleizig (UC-Berkeley), Joel Moss, (NIH-NHLBI), and Bonnie Ramsey (UW-Seattle). Representative publications:

