
Nancy M. Dahms, PhD
Emeritus Professor
Locations
- Biochemistry
BSB 307
Contact Information
Education
BS, Marquette University, 1980
Biography
Dr. Dahms received her Bachelor of Science degree from Marquette University in 1980 and her Doctorate degree in Biochemistry from the Johns Hopkins University School of Medicine in 1986. She was a postdoctoral fellow at Washington University School of Medicine from 1986-1989 where she isolated and characterized the cDNA clones for the receptors involved in targeting lysosomal enzymes to the lysosome. She joined the faculty of the Medical College of Wisconsin in 1989.
Research Experience
- Fabry Disease
- Glycoconjugates
- Lectins
- Lysosomal Storage Diseases
- Lysosomes
- Membrane Glycoproteins
- Membrane Proteins
- Organelles
- Quality control mechanisms in the secretory pathway
- Rat model of Fabry Disease
- Receptor, IGF Type 2
- Receptor, Mannose 6-phosphate
Leadership Positions
- Chair, Radiation Safety Committee 2000-2006
- Interim Chair, Department of Biochemistry 2009
- President Elect, Society for Glycobiology 2020
- President Faculty Council 2006-2007
- President, Society for Glycobiology 2021
- Sr Vice President Faculty Council 2005-2006
- Vice President Faculty Council 2004-2005
- Vice-Chairman, Curriculum and Evaluation Committee 2001-2002
Research Interests
Lysosomes carry out catabolism critical to many processes, including the disposal of abnormal proteins, cell survival, antigen processing, and inactivation of pathogenic organisms. Receptors deliver hydrolytic enzymes to lysosomes to generate functional lysosomes. Lysosomal storage diseases (LSDs) are caused by mutations in lysosomal proteins, mainly enzymes, that result in defective catabolism and substrate accumulation (‘storage’) of undegraded material within lysosomes, with lysosomal and cellular dysfunction leading to cell death and a shortened life span. As a group LSDs are among the most common genetic disorders in children, and their progressive and debilitating nature is due to their impact on multiple organ systems. Treatment is symptomatic for most LSDs, with only 11 out of the ~70 LSDs having FDA-approved therapies. Our goal is to define the molecular mechanism of receptor-mediated delivery of lysosomal enzymes to lysosomes, and thereby identify new strategies for the treatment of LSDs and other human diseases dependent upon lysosomal function.
Receptor Structural Biology & Protein-Carbohydrate Interactions
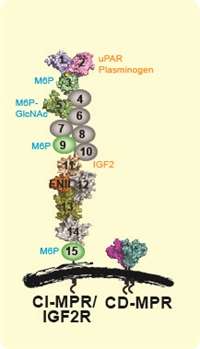
Two mannose 6-phosphate receptors (MPR), the cation-independent (CI-MPR) and the cation-dependent MPR (CD-MPR), bind a unique carbohydrate determinant, mannose 6-phosphate (M6P), on lysosomal enzymes’ N-glycans to mediate their delivery to lysosomes. CI-MPR, with its large extracellular region comprised of 15 contiguous domains, is the primary receptor responsible for lysosomal enzyme trafficking and CI-MPR forms the basis of enzyme replacement therapy used in the treatment of several LSDs. We have defined the glycan binding properties of the MPRs using biochemical, structural, cellular, and in vivo approaches. Our collaborative studies with Drs. Jung-Ja Kim and Brian Volkman using X-ray crystallography and NMR spectroscopy have provided the first and only three-dimensional structures reported to date of the carbohydrate binding sites of the MPRs.
Pathogenesis of lysosomal storage diseases (Fabry disease)
We are investigating the mechanisms underlying the diverse clinical symptoms experienced by patients with LSDs. Fabry disease, the most common LSD, is caused by a deficiency of the lysosomal enzyme, α-galactosidase A, which leads to glycosphingolipid accumulation in many cell types. We generated a rat model of Fabry disease, the first non-mouse model. Unlike Fabry mouse models, we show that Fabry rats recapitulate ocular, hearing, kidney, heart, and pain phenotypes experienced by Fabry patients and can be used to study disease mechanisms and test therapies.
Regulation of cell growth, survival, and differentiation
We are interested in the molecular mechanisms that regulate cell growth and differentiation. Our studies have focused on the role of growth factors (IGF2), proteases (plasminogen), and receptors (uPAR) in modulating cellular growth and differentiation of mammalian cells. The multifunctional CI-MPR (also known as IGF2R) also regulates the bioavailability of circulating IGF2 by targeting it to the lysosome, and in several animal models functions as a tumor suppressor of IGF2-dependent tumors. We have shown that the CI-MPR regulates fibroblast to myofibroblast differentiation and is critical for pancreatic beta cell survival.
Publications
-
Glycosphingolipids and their impact on platelet activity in a murine model of fabry disease.
(Kanack AJ, Prodoehl E, Ishihara-Aoki M, Aoki K, Dahms NM.) Sci Rep. 2024 Nov 27;14(1):29488 PMID: 39604471 PMCID: PMC11603304 SCOPUS ID: 2-s2.0-85210527022 11/28/2024
-
(Bohnsack RN, Misra SK, Liu J, Ishihara-Aoki M, Pereckas M, Aoki K, Ren G, Sharp JS, Dahms NM.) Sci Rep. 2024 Nov 06;14(1):26875 PMID: 39505925 PMCID: PMC11541866 SCOPUS ID: 2-s2.0-85208688601 11/07/2024
-
Fabry disease Schwann cells release p11 to induce sensory neuron hyperactivity.
(Waltz TB, Chao D, Prodoehl EK, Enders JD, Ehlers VL, Dharanikota BS, Dahms NM, Isaeva E, Hogan QH, Pan B, Stucky CL.) JCI Insight. 2024 Mar 07;9(8) PMID: 38646936 PMCID: PMC11141882 SCOPUS ID: 2-s2.0-85191335156 04/22/2024
-
Schwann cell release of p11 induces sensory neuron hyperactivity in Fabry disease.
(Waltz TB, Chao D, Prodoehl EK, Ehlers VL, Dharanikota BS, Dahms NM, Isaeva E, Hogan QH, Pan B, Stucky CL.) bioRxiv. 2023 May 28 PMID: 37292928 PMCID: PMC10245981 06/09/2023
-
(Yang C, Barbieri JT, Dahms NM, Chen C.) Biochemistry. 2022 Apr 05;61(7):616-624 PMID: 35285627 PMCID: PMC9817067 SCOPUS ID: 2-s2.0-85127145605 03/15/2022
-
Platelet and myeloid cell phenotypes in a rat model of Fabry disease.
(Kanack AJ, Aoki K, Tiemeyer M, Dahms NM.) FASEB J. 2021 Aug;35(8):e21818 PMID: 34320241 PMCID: PMC8341388 SCOPUS ID: 2-s2.0-85111530832 07/29/2021
-
(Miller JJ, Bohnsack RN, Olson LJ, Ishihara M, Aoki K, Tiemeyer M, Dahms NM.) Sci Rep. 2021 Apr 15;11(1):8213 PMID: 33859256 PMCID: PMC8050316 SCOPUS ID: 2-s2.0-85104287178 04/17/2021
-
Auditory brainstem responses in aging dark agouti rats.
(Beltrame AK, Dahms NM, Runge CL.) Biosci Rep. 2021 Feb 26;41(2) PMID: 33506259 PMCID: PMC7897922 SCOPUS ID: 2-s2.0-85102217469 01/29/2021
-
(Olson LJ, Misra SK, Ishihara M, Battaile KP, Grant OC, Sood A, Woods RJ, Kim JP, Tiemeyer M, Ren G, Sharp JS, Dahms NM.) Commun Biol. 2020 Sep 09;3(1):498 PMID: 32908216 PMCID: PMC7481795 SCOPUS ID: 2-s2.0-85090384984 09/11/2020
-
Progress in the understanding and treatment of Fabry disease.
(Miller JJ, Kanack AJ, Dahms NM.) Biochim Biophys Acta Gen Subj. 2020 Jan;1864(1):129437 PMID: 31526868 PMCID: PMC6981246 SCOPUS ID: 2-s2.0-85073720962 09/19/2019
-
Rats deficient in α-galactosidase A develop ocular manifestations of Fabry disease.
(Miller JJ, Aoki K, Reid CA, Tiemeyer M, Dahms NM, Kassem IS.) Sci Rep. 2019 Jun 28;9(1):9392 PMID: 31253878 PMCID: PMC6599056 SCOPUS ID: 2-s2.0-85068107461 06/30/2019
-
(Miller JJ, Aoki K, Mascari CA, Beltrame AK, Sokumbi O, North PE, Tiemeyer M, Kriegel AJ, Dahms NM.) FASEB J. 2019 Jan;33(1):418-429 PMID: 29979634 PMCID: PMC6629127 SCOPUS ID: 2-s2.0-85059237456 07/07/2018

