
Al W. Girotti, PhD
Emeritus Professor
Locations
- Biochemistry
BSB 359
Contact Information
Education
BS, Massachusetts Institute of Technology, 1959
Biography
Dr. Girotti received his Bachelor of Science degree in Biology from Massachusetts Institute of Technology in 1959 and his Doctorate degree in Biochemistry from the University of Massachusetts, Amherst in 1965. He was a Postdoctoral Research Associate at Cornell University Medical College (1965-1968) where he investigated the role of metal ions in ribonuclease activity. Dr. Girotti joined the faculty of the Biochemistry Department at the Medical College of Wisconsin in 1968.
Research Interests
Aerobic cells may experience oxidative stress damage if their enzymatic and non-enzymatic antioxidant defenses are overwhelmed by reactive oxygen species (ROS) generated by various endogenous and exogenous challenges. Unsaturated lipids in cell membranes and lipoproteins are prominent targets of ROS attack, undergoing peroxidative degradation with numerous structurally and functionally disruptive effects. Examples of free radical and non-radical ROS are shown in Scheme 1.
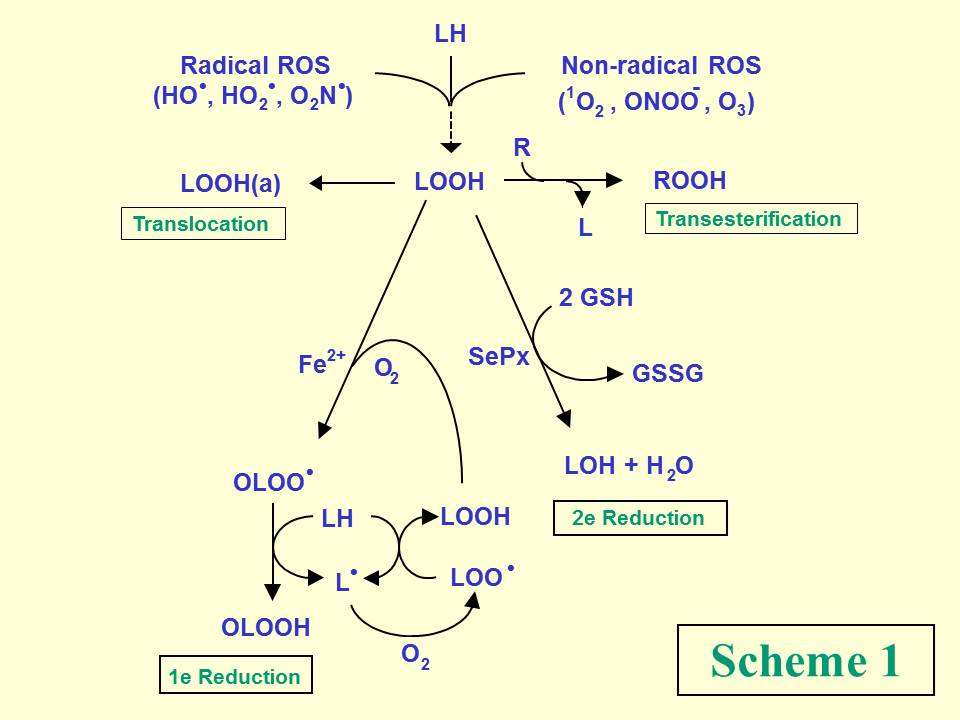
Among the many intermediates/products of lipid peroxidation, hydroperoxide species (LOOHs) are of special interest because of their relatively long lifetimes compared with free radical precursors or products. Under redox-constrained conditions, LOOHs can accumulate steadily with stress duration and may perturb membrane structure/function because of their relatively polar nature. However, in the presence of reductants and catalytic iron, LOOHs can undergo one-electron reduction with formation of oxyl (LO·) and epoxyallylic peroxyl (OLOO·) radicals, which exacerbate membrane damage by triggering chain peroxidation reactions (Scheme 1). Counteracting this is two-electron reductive detoxification catalyzed, for example, by glutathione-dependent selenoperoxidases, GPx4 (also known as PHGPx) being the most prominent isotype. Other LOOH pathways include inter-lipid transesterification and inter-membrane or membrane-lipoprotein translocation.
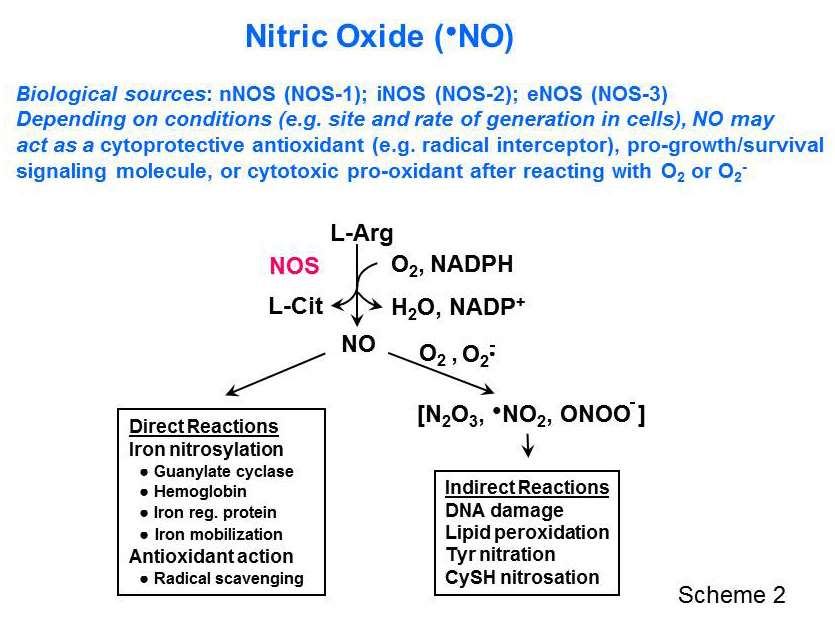
The Girotti group specializes in LOOH formation, turnover, and redox signaling activity, the latter currently attracting widespread biological and biomedical interest. Relatively low LOOH pressure may signal for upregulation of antioxidant proteins and activation of pro-growth transcription factors, whereas high LOOH pressure can signal for growth cessation and programmed cell death (apoptosis). Ongoing projects in the Girotti laboratory include the following: (a) Selenoperoxidase-mediated LOOH metabolism and how this modulates the pathologic as well as therapeutic effects of oxidative stress - as in antitumor photodynamic therapy (PDT), for example; (b) the biological ramifications of spontaneous or transfer protein-facilitated LOOH translocation between membranes or membranes and lipoproteins; pioneering studies of this phenomenon were carried out in the Girotti laboratory; (c) Protective effects of nitric oxide (NO) against cancer cell killing by PDT. Depending on its generation rate and location in cells, nitric oxide synthase (NOS)-derived NO can either be cytotoxic or cytoprotective (Scheme 2). In the latter category, our work has revealed that NO can (i) scavenge lipid-derived free radicals, (ii) induce antioxidant proteins such as heme oxygenase-1 and ferritin, or (iii) engage pro-survival/pro-growth signaling pathways that can compromise the effectiveness of PDT.
Publications
-
(Overchuk M, Choi AM, Rickard BP, Schnoor B, Ehrmann BM, Girotti AW, Zaharoff DA, Huang HC, O'Connell TM, Gomez SM, Rizvi I.) Cell Death Dis. 2025 Dec 10;16(1):899 PMID: 41372113 PMCID: PMC12722221 12/11/2025
-
(Girotti AW, Korbelik M.) Photochem Photobiol Sci. 2025 Dec;24(12):1983-1989 PMID: 41251969 11/18/2025
-
(Korbelik M, Heger M, Girotti AW.) J Lipid Res. 2025 Feb;66(2):100729 PMID: 39675508 PMCID: PMC11911859 SCOPUS ID: 2-s2.0-105000501101 12/16/2024
-
Tumor Lipid Signaling Involved in Hyperoxidative Stress Response: Insights for Therapeutic Advances.
(Korbelik M, Girotti AW.) J Cell Signal. 2025;6(2):39-47 PMID: 40458196 PMCID: PMC12129048 06/03/2025
-
(Girotti AW, Korytowski W.) Int J Mol Sci. 2024 May 23;25(11) PMID: 38891885 PMCID: PMC11171770 SCOPUS ID: 2-s2.0-85195836378 06/19/2024
-
(Pabisz P, Bazak J, Sabat M, Girotti AW, Korytowski W.) Cell Biochem Biophys. 2024 Mar;82(1):213-222 PMID: 37995086 PMCID: PMC10866752 11/23/2023
-
(Girotti AW, Bazak J, Korytowski W.) Int J Mol Sci. 2023 Jul 17;24(14) PMID: 37511317 PMCID: PMC10380283 07/29/2023
-
Trafficking of oxidative stress-generated lipid hydroperoxides: pathophysiological implications.
(Girotti AW, Korytowski W.) Free Radic Res. 2023 Feb;57(2):130-139 PMID: 37171212 PMCID: PMC10405667 SCOPUS ID: 2-s2.0-85159561103 05/12/2023
-
(Bazak J, Korytowski W, Girotti AW.) Crit Rev Oncog. 2023;28(1):15-25 PMID: 37824384 SCOPUS ID: 2-s2.0-85172468043 10/12/2023
-
(Girotti AW, Fahey JF, Korytowski W.) Crit Rev Oncol Hematol. 2022 Nov;179:103805 PMID: 36087851 SCOPUS ID: 2-s2.0-85137649262 09/11/2022
-
(Pabisz P, Bazak J, Girotti AW, Korytowski W.) Biochem Biophys Res Commun. 2022 Feb 05;591:82-87 PMID: 34999258 SCOPUS ID: 2-s2.0-85122320045 01/10/2022
-
The Negative Impact of Cancer Cell Nitric Oxide on Photodynamic Therapy.
(Fahey JM, Girotti AW.) Methods Mol Biol. 2022;2451:21-31 PMID: 35505007 SCOPUS ID: 2-s2.0-85129667244 05/04/2022

