
Blake Hill, PhD
Professor and Chair, Pharmaceutical Sciences, University of Colorado Anschutz; Adjunct Professor
Locations
- V20-3117 (UC Anschutz)
Contact Information
Education
Biography
Dr. Hill earned a PhD from Yale University (Biophysical Chemistry) in 1995 where he was a Frederick W. and Elsie L. Heyl graduate fellow. His postdoctoral training in protein design and engineering was completed at the University of Pennsylvania School of Medicine where he was awarded NIH and George W. Raiziss fellowships. Previously, he earned a bachelor's degree from Kalamazoo College followed by a stint at a major pharmaceutical company where he developed in vitro and in vivo models for oral bioavailability of peptide drugs. In 2000, Dr. Hill joined the faculty at Johns Hopkins University where began his NIH-funded research program in the molecular basis of mitochondrial homeostasis. He joined the faculty of the Medical College of Wisconsin in 2012 to pursue translational applications of his laboratory's basic research on the mechanism of mitochondrial homeostasis.
Research Experience
- Biochemistry
Research Interests
Defects in mitochondrial fission and fusion cause or contribute to human diseases including cancer, neuropathies, cardiomyopathies, and even death. Our goal is to understand molecular basis of these defects in order to identify new therapeutic routes for these diseases.
We determine how proteins interact with other biological macromolecules to control these basic membrane fission and fusion processes in healthy, diseased, and dying cells. 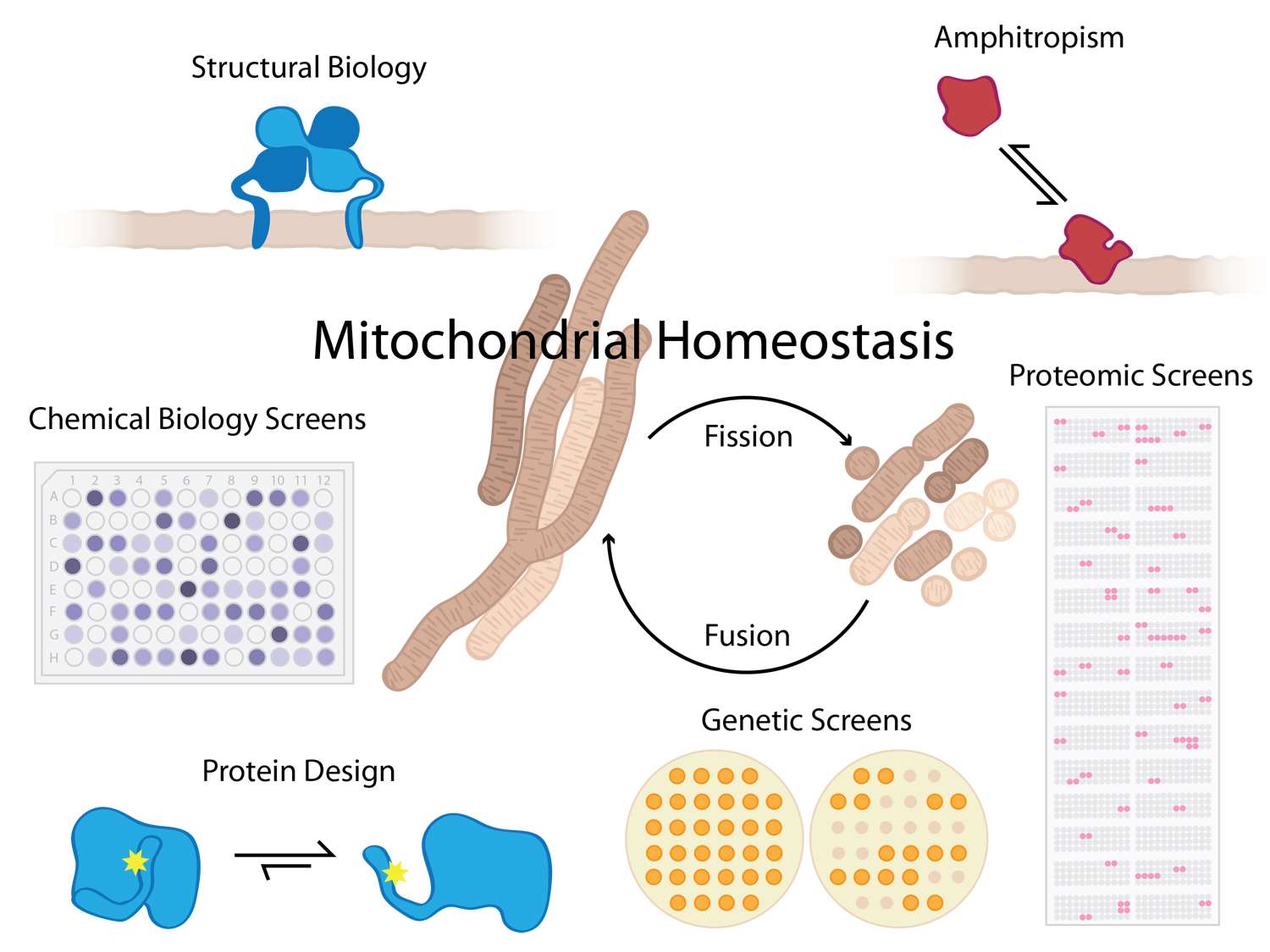 We strive to understand these interactions on a physicochemical level, with an eye for gleaning universal principles of protein chemistry including interactions with membrane bilayers that are fundamental to a wide variety of cellular processes.
We strive to understand these interactions on a physicochemical level, with an eye for gleaning universal principles of protein chemistry including interactions with membrane bilayers that are fundamental to a wide variety of cellular processes.
A key feature of some proteins that affect mitochondrial homeostasis is the structural transformation from soluble to membrane-bound conformations, a phenomenon referred to as amphitropism. Associating with, or dissociating from, a membrane (i.e. amphitropism) has significant functional consequences for numerous biological processes: it can affect enzymatic activity (CCT, PLC), can promote changes in organelle and cell morphology (MinD, dynamins), or can act as a regulatory switch in various signaling cascades (PKC, ESCRTs). However, neither what drives proteins to reversibly interact with membranes nor how this function controls biological outcomes are clearly understood. These interactions are likely governed by evolutionarily conserved mechanisms that are still being determined and is one focus of our efforts.
Towards these goals, we use a wide range of tools including genetic, cell biological, biochemical, and biophysical methods including NMR spectroscopy and x-ray crystallography for protein structure determination. Please visit the Hill Lab website for more information.

