
Ravi P. Misra, PhD
Associate Provost, Accreditation and Program Development; Professor
Locations
- Biochemistry
M1420/BSB 353
Contact Information
Education
BA, Rutgers College, 1979
Biography
Dr. Misra obtained his Bachelor of Arts degree in Microbiology from Rutgers College in New Jersey in 1979 and his Doctorate degree in Biochemistry from New York University School of Medicine in 1988. He was a Postdoctoral Research Fellow at Harvard Medical School where he studied the molecular control of growth factor induced transcriptional regulation of the c-Fos proto-oncogene. Dr. Misra joined the faculty of the Biochemistry Department at the Medical College of Wisconsin in 1993.
Research Interests
My major research interest is to understand the biochemical mechanisms that control expression of genes at the transcriptional level. Our current work concerns (1) understanding the mechanisms by which the Serum Response Factor (SRF) mediates gene expression during early cardiogenesis, and (2) understanding mechanisms controlling the in vivo expression of the SRF gene itself during early development.
One of the overall objectives of our research is to elucidate underlying molecular mechanisms involved in cardiac function and heart formation. Congenital cardiovascular anomalies are the most common form of human birth defect with a recorded instance of 1 per 200 live births per year in North America. There is therefore considerable interest in understanding the molecular and genetic bases of these diseases. Underlying congenital heart defects is the complexity of cardiovascular development. Proper heart development requires the precise expression at the temporal and spatial level of a complex cast of structural and regulatory proteins. Alterations in either the function of these proteins, their time or location of expression, or their abundance relative to other critical proteins can have drastic consequences for cardiac development ranging from embryonic lethality to functional malformations of the heart that can lead to significant cardiomyopathies. 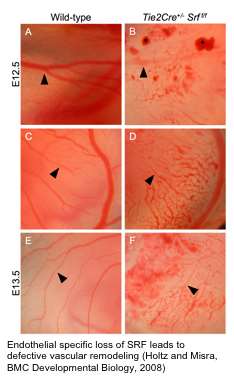 One way in which proteins can be become misexpressed is by aberrant regulation Misra Image 1of expression of the genes that encode them. It is therefore important to understand the mechanisms that regulate normal expression of these proteins during formation of the heart. However, due to the significant limitations of the current technologies used to study embryonic gene regulation and protein function this has been difficult to address. In our studies we are using a novel sophisticated mouse transgenic approach to study the molecular mechanisms involved in controlling regulation of a transcriptional regulatory protein, the serum response factor (SRF).
One way in which proteins can be become misexpressed is by aberrant regulation Misra Image 1of expression of the genes that encode them. It is therefore important to understand the mechanisms that regulate normal expression of these proteins during formation of the heart. However, due to the significant limitations of the current technologies used to study embryonic gene regulation and protein function this has been difficult to address. In our studies we are using a novel sophisticated mouse transgenic approach to study the molecular mechanisms involved in controlling regulation of a transcriptional regulatory protein, the serum response factor (SRF).
Serum response factor (SRF), is a highly evolutionarily conserved member of the MADS (MCM1, Agamous and Deficiens, SRF) box family of transcription factors, that is a critical regulator of cardiac development and function. SRF regulates various cardiac and skeletal muscle specific genes necessary for normal heart development and function, including the cardiac and skeletal actin, dystrophin, myosin light chain, and atrial natriuretic peptide genes. Consistent with this, cardiac specific overexpression of SRF in transgenic animals results in reinduction of an embryonic program of gene expression that can lead to dramatic cardiac hypertrophic and myopathic phenotypes that mimic those observed during the initial development of congestive heart failure in humans. Cardiac specific loss of SRF leads to embryonic death due to vascular insufficiency, and consistent with this we have shown that in cardiomyocytes SRF is an essential coordinator of cardiomyocyte function due to its ability to regulate expression of numerous genes involved in multiple and disparate levels of sarcomeric function and assembly.
A major focus of our lab is to understand the genetic regulation of coronary vascular development. We and others have shown that SRF is important for vascular Misra Image 2development and function in the heart. SRF plays a critical role in vascular smooth muscle gene expression and function.
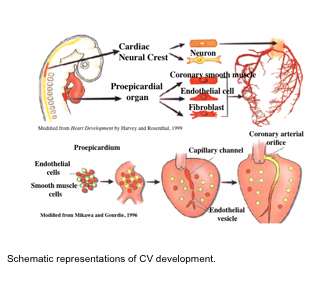 We have also shown SRF is important for endothelial cell function and that endothelial specific loss of SRF disrupts vascular remodeling. In higher vertebrates, development of the coronary vascular (CV) system is dependent on a group of multipotent cells located in the proepicardium (PE). The PE is a transient embryonic structure located just below the developing A/V canal of the early heart. The PE is of central importance in CV development. Genetic labeling experiments in avian systems have established that this generative tissue contributes to all the vascular cells of the heart. In mouse, at around embryonic day (ED) 9.5 cells from the PE undergo an epithelial/mesenchymal-like transformation and migrate to invest the surface of the heart and form the epicardium. Epicardial-derived cells subsequently diversify into subepicardial mesenchymal cells (SEMCs), from which arises the cardiac fibroblasts, pericytes, as well as the endothelial and smooth muscle cells that comprise the coronary vasculature. A significant amount of work in avian systems has established the central importance of the PE for CV development. However, largely due to a lack of definitive PE specific markers and the relatively diminutive size and transient nature of the PE, there still remains much to be understood about how PE cells ultimately form the CV. An outstanding question that has direct therapeutic implication, is whether the PE can serve as an efficacious source of cells to effect directed vascular repair after cardiac injury.
We have also shown SRF is important for endothelial cell function and that endothelial specific loss of SRF disrupts vascular remodeling. In higher vertebrates, development of the coronary vascular (CV) system is dependent on a group of multipotent cells located in the proepicardium (PE). The PE is a transient embryonic structure located just below the developing A/V canal of the early heart. The PE is of central importance in CV development. Genetic labeling experiments in avian systems have established that this generative tissue contributes to all the vascular cells of the heart. In mouse, at around embryonic day (ED) 9.5 cells from the PE undergo an epithelial/mesenchymal-like transformation and migrate to invest the surface of the heart and form the epicardium. Epicardial-derived cells subsequently diversify into subepicardial mesenchymal cells (SEMCs), from which arises the cardiac fibroblasts, pericytes, as well as the endothelial and smooth muscle cells that comprise the coronary vasculature. A significant amount of work in avian systems has established the central importance of the PE for CV development. However, largely due to a lack of definitive PE specific markers and the relatively diminutive size and transient nature of the PE, there still remains much to be understood about how PE cells ultimately form the CV. An outstanding question that has direct therapeutic implication, is whether the PE can serve as an efficacious source of cells to effect directed vascular repair after cardiac injury.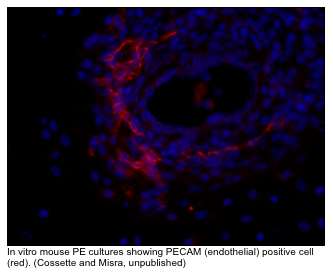
In my laboratory we have recently begun to address the function of the PE in CV development in mouse model systems by establishing genetic tools that can be used to elucidate the function of subsets of PE-derived vascular cells. Notably, we have identified an evolutionarily conserved DNA enhancer element, termed the PE enhancer (ePE), which confers a vasculogenic expression pattern to reporter genes.
Currently, the work in the lab involves using cellular, molecular, and transgenic approaches to study various aspects of early coronary vascular differentiation. We are focusing on PE-development, transcriptional regulation of endothelial cell differentiation and function, and identifying PE-derived vascular stem cells.
Publications
-
Nogo-B receptor deficiency causes cerebral vasculature defects during embryonic development in mice.
(Rana U, Liu Z, Kumar SN, Zhao B, Hu W, Bordas M, Cossette S, Szabo S, Foeckler J, Weiler H, Chrzanowska-Wodnicka M, Holtz ML, Misra RP, Salato V, North PE, Ramchandran R, Miao QR.) Dev Biol. 2016 Feb 15;410(2):190-201 PMID: 26746789 PMCID: PMC4767500 SCOPUS ID: 2-s2.0-84957847497 01/10/2016
-
Epicardial GATA factors regulate early coronary vascular plexus formation.
(Kolander KD, Holtz ML, Cossette SM, Duncan SA, Misra RP.) Dev Biol. 2014 Feb 01;386(1):204-15 PMID: 24380800 PMCID: PMC3962664 01/02/2014
-
The identification of different endothelial cell populations within the mouse proepicardium.
(Cossette S, Misra R.) Dev Dyn. 2011 Oct;240(10):2344-53 PMID: 21932312 PMCID: PMC3275641 09/21/2011
-
(Holtz ML, Misra RP.) BMC Dev Biol. 2011 Mar 14;11:18 PMID: 21401944 PMCID: PMC3065428 03/16/2011
-
The role of serum response factor in early coronary vasculogenesis.
(Misra RP.) Pediatr Cardiol. 2010 Apr;31(3):400-7 PMID: 20091302 PMCID: PMC3866703 01/22/2010
-
(Sun K, Battle MA, Misra RP, Duncan SA.) Hepatology. 2009 May;49(5):1645-54 PMID: 19205030 PMCID: PMC2810404 SCOPUS ID: 2-s2.0-66149117384 02/11/2009
-
(Holtz ML, Misra RP.) BMC Dev Biol. 2008 Jun 20;8:65 PMID: 18570667 PMCID: PMC2442838 06/24/2008
-
(Major ML, Cheung HS, Misra RP.) Biochem Biophys Res Commun. 2007 Apr 13;355(3):654-60 PMID: 17307136 PMCID: PMC1855205 02/20/2007
-
(Balza RO Jr, Misra RP.) J Biol Chem. 2006 Mar 10;281(10):6498-510 PMID: 16368687 12/22/2005
-
(Nelson TJ, Balza R Jr, Xiao Q, Misra RP.) J Mol Cell Cardiol. 2005 Sep;39(3):479-89 PMID: 15950986 06/14/2005
-
Restricted inactivation of serum response factor to the cardiovascular system.
(Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO Jr, Xiao Q, Weiler H, Ginty DD, Misra RP.) Proc Natl Acad Sci U S A. 2004 Dec 07;101(49):17132-7 PMID: 15569937 PMCID: PMC535359 SCOPUS ID: 2-s2.0-10344236515 12/01/2004
-
(Nelson TJ, Duncan SA, Misra RP.) Circ Res. 2004 Apr 30;94(8):1059-66 PMID: 15001533 03/06/2004

