
Stephanie Olivier-Van Stichelen, PhD
Associate Professor, Biochemistry; Secondary Faculty in Neurosurgery and Obstetrics & Gynecology
Locations
- Biochemistry
BSB 355
Contact Information
General Interests
Education
Post-doctoral training, National Institute of health, Bethesda, MD
Biography
Dr. Olivier-Van Stichelen received her PhD in Biochemistry from the University of Lille, France in 2012, where she studied the nutrient-sensing O-GlcNAcylation in colorectal cancer development. After completing her degree, she was appointed as a post-doctoral Fellow at the National Institute of Health, Bethesda, MD, USA. In this lab, Dr. Olivier-Van Stichelen worked on different aspects of O-GlcNAcylation during development, including X-inactivation of the O-GlcNAc Transferase gene, brain O-GlcNAcase function in mouse KO models and the impact of sugar and artificial sweeteners consumption during pregnancy on the development of metabolic homeostasis.
Dr. Olivier-Van Stichelen established her lab at the Medical College of Wisconsin in 2019 at the crossroad of sweeteners, pregnancy, development, and metabolism. Notably, her lab has developed several mouse models for studying O-GlcNAcylation and created the O-GlcNAc database, the reference catalog of O-GlcNAcylated proteins for all species. Some of the current lab projects include studies of (i) O-GlcNAcylation in the pathophysiology of the pituitary gland, (ii) O-GlcNAc transferase’s function in the sexual dimorphism of Gestational Diabetes, and (iii) artificial sweeteners’ impact on detoxification transporters.
In The News
How Natural and Artificial Sweeteners Affect the Body
Wisconsin Public Radio
Fit For You: Artificial Sweeteners
WUWM 89.7
Access the O-GlcNAc Database
A catalog of O-GlcNAcylated proteins, their O-GlcNAc sites, and corresponding references
Research Experience
- Acetylglucosamine
- Animals
- beta Catenin
- beta-N-Acetylhexosaminidases
- Blotting, Western
- Diet
- Fasting
- Female
- Glucose
- Glycosylation
- Intestinal Mucosa
- Mice
Research Interests
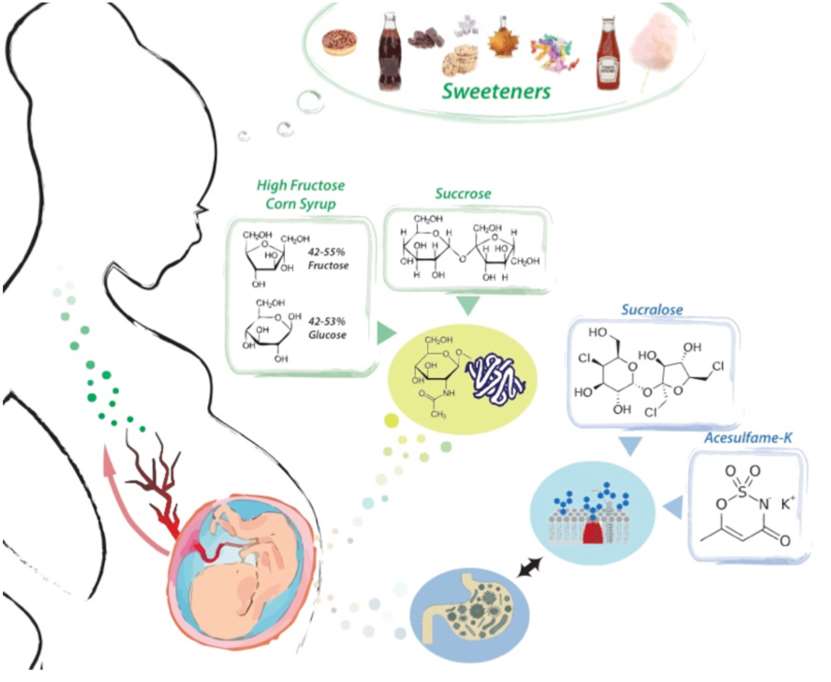
Due to the global trend of growing sweetener consumption, determining the interplay between diet and pre- and post-natal development is emerging as a critical area for research. Currently, the average American eats around 22 teaspoons of added sugar every day (30 sugar cubes/day hidden in foods). This modern glucose-rich diet correlates with an increase in the prevalence of obesity, diabetes and others metabolic syndromes. Moreover, the effort to reduce sugar consumption has led people to consume more non-caloric sweeteners (Aspartame, Sucralose, Acesulfame-K...). While they appear healthier for glucose homeostasis than a high carbohydrate diet, recent studies have shown that artificial sweeteners impact glucose metabolism as well as gut microbiota, rising questions about their excessive use.
Therefore, understanding what happens when caloric and non-caloric sweeteners are metabolized is of utmost importance for public health and the focus of my research group.
Nutrient-dependent O-GlcNAc cycling in development and disease
O-GlcNAcylation is one of the key components of diet-responsive signaling. This unique glucose rheostat is a ubiquitous and dynamic glycosylation of intracellular proteins with approximately 1000 modified proteins described to date. Two key enzymes drive O-GlcNAc cycling: The O-GlcNAc transferase (OGT) adds the modification and the O-GlcNAcase (OGA) removes it. Although many studies have focused on the decrease or complete absence of O-GlcNAc cycling by modulating the expression or activity of OGT, only a few studies have targeted hyper-O-GlcNAcylation by disturbing OGA. Because this post-translational modification is directly dependent on glucose input, depleting OGA creates an artificial and constant hyperglycemia-induced O-GlcNAcylation state. Using Oga and Ogt knockout (KO) cellular and mouse models, we can decipher the impact of high carbohydrate diet on embryonic development.
Non-Nutritive Sweeteners in pregnancy and lactation
Part of my lab is interested in understanding the impact of Non-Nutritive Sweetener (NNS) consumption through pregnancy and lactation. Although, NNS have been found in mother’s milk and in placental blood circulation, no study has focused on the fundamental effect of those non-caloric sweeteners on the developing organism. Among the impacts described in adults are changes in intestinal hormonal secretion, glucose metabolism and most fascinating, re- duction of the gut microbiota. Nevertheless, the fundamental mechanisms of those changes are far from understood. Glycoproteins found on the surface of the intestinal epithelium define the glycocalyx and are an essential mammalian mechanism of communication with the gut microbiome. Their reciprocal relationship with the gut microbiome regulates not only nutrient breakdown, and food absorption, but also infection. We are convinced that by altering both microbiome and the detoxification process, NNS exposure in early life will impact metabolic homeostasis later in life.
Publications
-
(Vanauberg D, Schulz C, Raab S, Fuentes-GarcÍa G, Cadart E, Lemaire Q, Olivier-Van Stichelen S, Bray F, Brysbaert G, Rossez Y, Hardivillé S, Lefebvre T.) J Biol Chem. 2025 Aug;301(8):110497 PMID: 40684943 PMCID: PMC12362114 SCOPUS ID: 2-s2.0-105012995897 07/21/2025
-
Non-nutritive sweeteners in food-drug interactions: An overview of current evidence.
(Danner L, Kroenke K, Olivier-Van Stichelen S.) Mol Pharmacol. 2025 May;107(5):100035 PMID: 40318386 SCOPUS ID: 2-s2.0-105003965121 05/04/2025
-
O-GlcNAcylation in Endocrinology: The Sweet Link.
(Knier AS, Olivier-Van Stichelen S.) Endocrinology. 2025 Apr 22;166(6) PMID: 40209111 PMCID: PMC12013285 SCOPUS ID: 2-s2.0-105003433048 04/10/2025
-
The O-GlcNAc database: introducing new features and tools developed from community feedback.
(Malard F, Massman L, Campagne S, Olivier-Van Stichelen S.) Anal Bioanal Chem. 2025 Feb;417(5):879-884 PMID: 39379619 PMCID: PMC12105936 SCOPUS ID: 2-s2.0-85205887981 10/09/2024
-
The diverging role of O-GlcNAc transferase in corticotroph and somatotroph adenomas.
(Gonzalez R, Massman L, Ho S, Luna S, Cheok S, Liang B, Mrachek K, Coss D, Ioachimescu AG, Zwagerman N, Olivier-Van Stichelen S.) Pituitary. 2024 Oct;27(5):577-589 PMID: 39066842 PMCID: PMC12147819 SCOPUS ID: 2-s2.0-85200049784 07/28/2024
-
Studying the O-GlcNAcome of human placentas using banked tissue samples.
(Luna S, Malard F, Pereckas M, Aoki M, Aoki K, Olivier-Van Stichelen S.) Glycobiology. 2024 Apr 10;34(4) PMID: 38253038 PMCID: PMC11005170 SCOPUS ID: 2-s2.0-85190109720 01/23/2024
-
O-GlcNAcylation regulates OTX2's proteostasis.
(Wulff-Fuentes E, Boakye J, Kroenke K, Berendt RR, Martinez-Morant C, Pereckas M, Hanover JA, Olivier-Van Stichelen S.) iScience. 2023 Nov 17;26(11):108184 PMID: 38026167 PMCID: PMC10661118 11/29/2023
-
(Danner L, Malard F, Valdes R, Olivier-Van Stichelen S.) Nutrients. 2023 Feb 23;15(5) PMID: 36904118 PMCID: PMC10005754 SCOPUS ID: 2-s2.0-85149772752 03/12/2023
-
O-GlcNAcylation regulates OTX2’s proteostasis
(Wulff-Fuentes E, Boakye J, Kroenke K, Berendt RR, Martinez-Morant C, Pereckas M, Hanover JA, Olivier-Van Stichelen S.) Iscience. 17 November 2023;26(11) SCOPUS ID: 2-s2.0-85175489896 11/17/2023
-
O-GlcNAc transferase contributes to sex-specific placental deregulation in gestational diabetes.
(Cui Y, Cruz M, Palatnik A, Olivier-Van Stichelen S.) Placenta. 2023 Jan;131:1-12 PMID: 36442303 PMCID: PMC9839643 SCOPUS ID: 2-s2.0-85142764152 11/29/2022
-
The O-GlcNAc cycling in neurodevelopment and associated diseases.
(Wenzel DM, Olivier-Van Stichelen S.) Biochem Soc Trans. 2022 Dec 16;50(6):1693-1702 PMID: 36383066 PMCID: PMC10462390 SCOPUS ID: 2-s2.0-85144232323 11/17/2022
-
(Sheikh MO, Capicciotti CJ, Olivier-Van Stichelen S.) Front Mol Biosci. 2022;9:1012485 PMID: 36158575 PMCID: PMC9493436 09/27/2022

