
Calvin B. Williams, MD, PhD
Professor, Pediatrics and Microbiology & Immunology; Associate Dean of Research; Associate Director, Medical Scientist Training Program; Chief Scientific Officer, Children's Wisconsin Research Institute; Chief, Pediatric Rheumatology; Vice Chair, Research, Pediatrics
Contact Information
General Interests
Education
Leadership Positions
- Chief Scientific Officer, Children's Research Institute
- Professor of Pediatrics, Microbiology and Immunology
- Section Chief, Pediatric Rheumatology
Research Interests

The broad, long-term objective of research in my laboratory is to establish the mechanisms that promote T cell development in the thymus and maintain T cell tolerance in the periphery. We place special emphasis on role of Foxp3 regulatory T (Treg) cells, and have developed a number of mouse models that are widely used to study Treg cell function. This body of work has contributed to our understanding of T cell receptor antagonism as potent mechanism of peripheral tolerance (1), the mechanisms of Treg cell-mediated suppression in models of inflammatory autoimmune disease (2), the role of Foxp3 in Treg cell development (3), and the identification of “induced” Treg (iTreg) cells as an essential regulatory subset required for mucosal tolerance (4). Current work is focused on discovering the mechanisms that control the inducible components of mucosal tolerance, including the iTreg-Th17 cell axis (Figure 1).
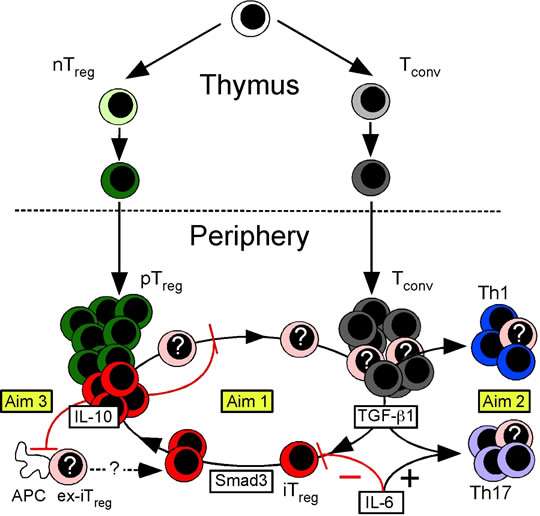
Figure 1. Model of Treg cell development. The nTreg cell population (green) develops as a distinct lineage in the thymus. In the periphery, TGF-b1 induces Tconv cells to become iTreg cells (red) or Th17 cells (purple). The peripheral Treg (pTreg) cell pool is therefore comprised of both iTreg and nTreg cells. IL-6 promotes production of Th17 cells while blocking iTreg cell formation. Smad3 promotes Foxp3 expression and stability. In some circumstances, iTreg cells are unstable and lose Foxp3 expression (ex-Foxp3+ iTreg or “ex-iTreg” cells, pink). These ex-iTreg cells may become Th1 (blue) or Th17 cells, or reacquire Foxp3 and cycle back into the iTreg pool. IL-10 produced by iTreg cells functions in an autocrine feedback loop to promote iTreg cell transcriptional and phenotypic stability. The activation of ex-iTreg cells and their migration from the mesenteric lymph nodes to the intestinal mucosa is also specifically prevented by iTreg cell produced IL-10. In Current work in the lab is divided into three Aims. In Aim 1, we will determine how the iTreg cell niche is created and maintained. In Aim 2 we will examine the fate of ex-iTreg cells, and in Aim 3 we will show that iTreg cell produced IL-10 has both shared and unique roles. in gastrointestinal tolerance.
Publications
-
(Sabbagh SE, Haribhai D, Gershan JA, Verbsky J, Nocton J, Yassai M, Naumova EN, Hammelev E, Dasgupta M, Yan K, Gorski J, Williams CB.) Front Immunol. 2024;15:1446946 PMID: 38962010 PMCID: PMC11220386 SCOPUS ID: 2-s2.0-85197728513 07/04/2024
-
(Sabbagh SE, Haribhai D, Gershan JA, Verbsky J, Nocton J, Yassai M, Naumova EN, Hammelev E, Dasgupta M, Yan K, Gorski J, Williams CB.) Front Immunol. 2024;15:1306490 PMID: 38873594 PMCID: PMC11169902 SCOPUS ID: 2-s2.0-85195642328 06/14/2024
-
BATF is Required for Treg Homeostasis and Stability to Prevent Autoimmune Pathology.
(Khatun A, Wu X, Qi F, Gai K, Kharel A, Kudek MR, Fraser L, Ceicko A, Kasmani MY, Majnik A, Burns R, Chen YG, Salzman N, Taparowsky EJ, Fang D, Williams CB, Cui W.) Adv Sci (Weinh). 2023 Oct;10(28):e2206692 PMID: 37587835 PMCID: PMC10558681 SCOPUS ID: 2-s2.0-85168114370 08/17/2023
-
(Cai Y, Schroeder JA, Jing W, Gurski C, Williams CB, Wang S, Dittel BN, Shi Q.) Front Immunol. 2022;13:1029356 PMID: 36389708 PMCID: PMC9647046 SCOPUS ID: 2-s2.0-85141698554 11/18/2022
-
(Li J, Chen J, Schroeder JA, Hu J, Williams CB, Shi Q.) Mol Ther Nucleic Acids. 2021 Mar 05;23:719-730 PMID: 33575117 PMCID: PMC7851450 02/13/2021
-
(Leong JY, Chen P, Yeo JG, Ally F, Chua C, Nur Hazirah S, Poh SL, Pan L, Lai L, Lee ESC, Bathi LDT, Arkachaisri T, Lovell D, Albani S, Pediatric Rheumatology Collaborative Study Group.) Ann Rheum Dis. 2019 Dec;78(12):1712-1721 PMID: 31540934 PMCID: PMC6900250 09/22/2019
-
Regulatory T Cells Control PF4/Heparin Antibody Production in Mice.
(Zheng Y, Zhu W, Haribhai D, Williams CB, Aster RH, Wen R, Wang D.) J Immunol. 2019 Oct 01;203(7):1786-1792 PMID: 31471526 PMCID: PMC6944762 SCOPUS ID: 2-s2.0-85072761081 09/01/2019
-
Thymic regulatory T cells arise via two distinct developmental programs.
(Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, Ruscher R, Huang W, Proekt I, Miller CN, Hekim C, Jeschke JC, Aggarwal P, Broeckel U, LaRue RS, Henzler CM, Alegre ML, Anderson MS, August A, Marson A, Zheng Y, Williams CB, Farrar MA.) Nat Immunol. 2019 Feb;20(2):195-205 PMID: 30643267 PMCID: PMC6650268 SCOPUS ID: 2-s2.0-85059964938 01/16/2019
-
Age-Based Dynamics of a Stable Circulating Cd8 T Cell Repertoire Component.
(Naumova EN, Yassai MB, Demos W, Reed E, Unruh M, Haribhai D, Williams CB, Naumov YN, Gorski J.) Front Immunol. 2019;10:1717 PMID: 31447830 PMCID: PMC6691812 SCOPUS ID: 2-s2.0-85071983500 08/27/2019
-
(Lovell DJ, Johnson AL, Huang B, Gottlieb BS, Morris PW, Kimura Y, Onel K, Li SC, Grom AA, Taylor J, Brunner HI, Huggins JL, Nocton JJ, Haines KA, Edelheit BS, Shishov M, Jung LK, Williams CB, Tesher MS, Costanzo DM, Zemel LS, Dare JA, Passo MH, Ede KC, Olson JC, Cassidy EA, Griffin TA, Wagner-Weiner L, Weiss JE, Vogler LB, Rouster-Stevens KA, Beukelman T, Cron RQ, Kietz D, Schikler K, Schmidt KM, Mehta J, Wahezi DM, Ting TV, Verbsky JW, Eberhard BA, Spalding S, Chen C, Giannini EH.) Arthritis Rheumatol. 2018 Sep;70(9):1508-1518 PMID: 29604189 PMCID: PMC6115300 SCOPUS ID: 2-s2.0-85052490977 04/01/2018
-
(Jeschke JC, Mayne CG, Ziegelbauer J, DeCiantis CL, Singh S, Kumar SN, Suchi M, Iwakura Y, Drobyski WR, Salzman NH, Williams CB.) Mucosal Immunol. 2018 Jul;11(4):1127-1137 PMID: 29728642 PMCID: PMC6571016 SCOPUS ID: 2-s2.0-85046438531 05/08/2018
-
(Luo X, Chen J, Schroeder JA, Allen KP, Baumgartner CK, Malarkannan S, Hu J, Williams CB, Shi Q.) Front Immunol. 2018;9:1950 PMID: 30237796 PMCID: PMC6136275 SCOPUS ID: 2-s2.0-85053056830 09/22/2018

