Science Perseveres: Because Knowledge Changes Lives

Science doesn’t sleep. At any given moment, scientists somewhere in the world are eagerly pursuing new insight into the forces and phenomena that shape society and influence health. Only a catastrophic event can slow down the wheels of progress as they move forward in the global search for new knowledge.
The emergence and spread of the SARSCoV-2 virus that created the COVID-19 pandemic led to just such a historically rare, deadly and disruptive event for humanity – one that continues to challenge us today. In the US and at the Medical College of Wisconsin (MCW), the need for physical distancing required leaders to shut down a significant amount of scientific experimentation in the spring and summer of 2020. This unprecedented step had not been required during prior national and international crises.
Despite these circumstances and obstacles, MCW scientists have persevered. They have shown the ability to adapt and be both safe and productive as understanding of the COVID-19 pandemic and institutional policies evolved, and as vaccines became available to aid in the recovery of the research mission. MCW research administrators have pushed forward on important improvements to scientific infrastructure while investigators have continued to advance their experiments. The determination of all during a very difficult time continues to sustain MCW’s growth as one of the nation’s leading centers for innovation and discovery.
Hibernation and Recovery
MCW shut down most labs and studies in late March 2020.
“I vividly remember leaving on the last day the research enterprise was open,” recalls Cecilia “Cece” Hillard, PhD ’83, G. Frederick Kasten, Jr. Endowed Chair in Parkinson’s Disease Research, professor of pharmacology and toxicology, associate dean for research and director of MCW’s Neuroscience Research Center. “We called it hibernating the labs – and it definitely felt like entering hibernation. It was eerie, incredibly quiet and, honestly, quite chilling. And we didn’t know when we’d be back.”
Dr. Hillard and other leaders in the research mission immediately went to work creating new health and safety guidelines that could support a gradual return from hibernation and a progressive increase in research activity over time. MCW began an initial phase of limited laboratory research activity in May 2020, which included guidelines such as limiting the number of people in laboratories and requiring physical distancing, facial coverings and decontamination of workspaces and shared equipment.
In June 2020, MCW began to allow the resumption of a select number of clinical trials and other human subjects research studies. Throughout the summer and fall, the institution moved through several phases of increasing laboratory and clinical research levels so that health and safety could be assessed at each stage of the plan.
“Looking back, I continue to believe we recovered about as well as we could have given what we knew,” says Ann Nattinger, MD, MPH, Lady Riders Professor of Breast Cancer Research at MCW, associate provost for research and professor of medicine. “The vaccine helped us accelerate our reactivation and running our own vaccination clinic enabled our scientists and support staff to get vaccinated as soon as it was possible within the state’s guidelines.” Dr. Nattinger shares that everyone in the research mission felt better about being on the Milwaukee campus after this step, and it allowed MCW to provide more flexibility to investigators to staff their labs. Clinical research teams also were able to increase the pace of experimentation.
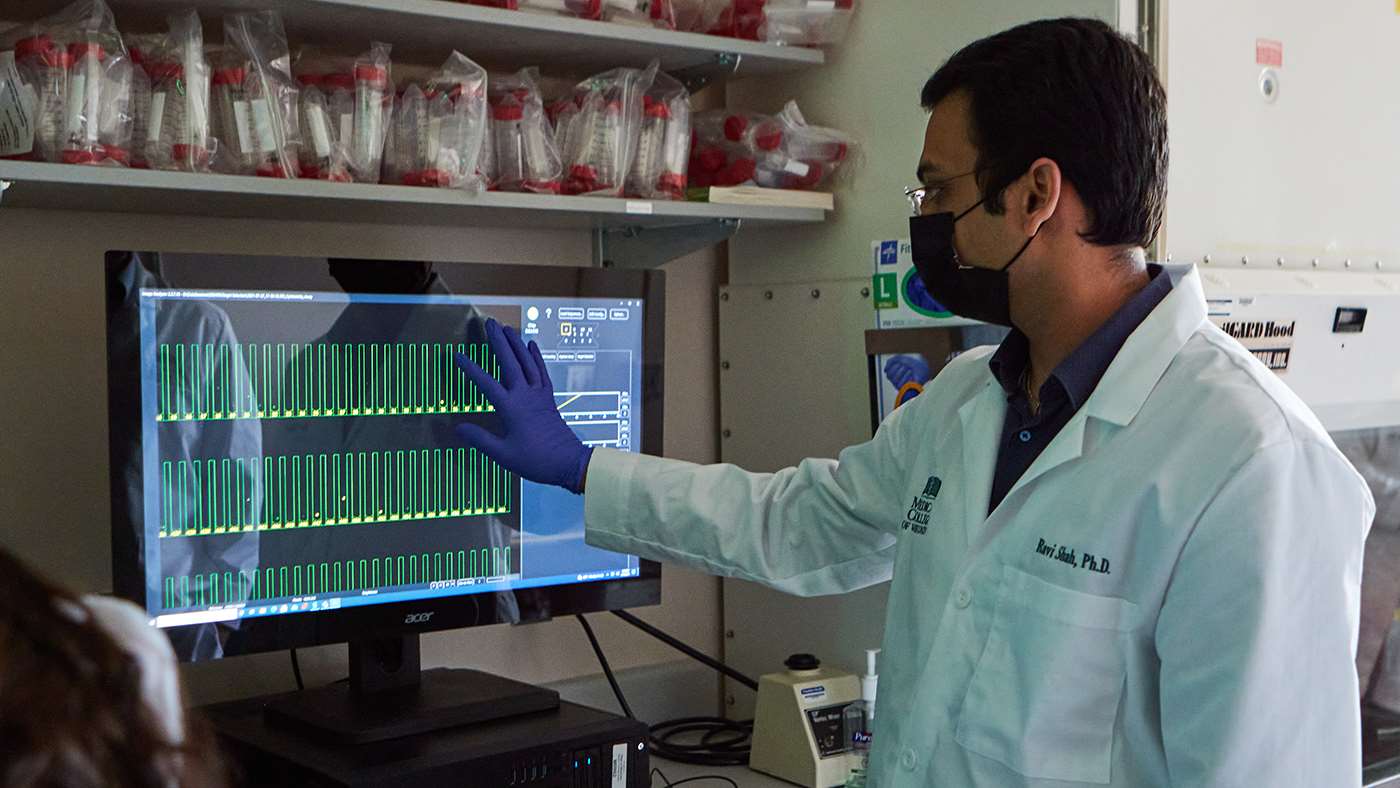
Adapting in the Labs
Initially, MCW’s lab-based researchers largely turned their focus to what they could accomplish through virtual work. This included writing and submitting manuscripts to scholarly journals and proposals for grants and other funding opportunities using data they had generated before the pandemic. Labs also invested additional time in reviewing and discussing scientific literature to continue furthering the education and career development of postdoctoral fellows and graduate students.
“People could have allowed fear and frustration to prevent them from doing their best work,” notes Dr. Hillard. “Instead, our investigators and their teams rallied around each other and found every possible way to make meaningful contributions.”
As MCW policies for the research mission evolved during the pandemic to allow labs to run experiments by spreading out workers over multiple shifts, scientists found that MCW’s Biomedical Resource Center was ready to help them restart paused projects or jump-start new ones. The center’s technicians and veterinary staff worked tirelessly to sustain a high-quality animal research environment with state-of-the-art animal husbandry and veterinary medical care.
“I’m really proud of the ingenuity and hard work that has been evident in the labs,” adds Dr. Nattinger. “Everyone has adjusted and found ways to move their research forward. Recently, we’ve seen a substantial increase in applications for external research funding. All of these submissions require preliminary data, so it is clear to me that individuals have found ways to adapt and successfully complete important experiments.”
From Bench to Bedside
From the earliest meetings about how to handle MCW clinical research during the pandemic, leaders agreed that some studies needed to continue with protections for patients, research personnel and healthcare staff.
“In the Cancer Center, we focused on Phase I trials, which test new, promising treatments for people who have no other therapeutic options,” says James “Jim” Thomas, MD ’91, PhD ’89, GME ’95, professor of medicine (hematology and oncology) and associate director of translational research for the MCW Cancer Center. To be able to offer these and other studies determined to be essential, Dr. Thomas and his colleagues developed modified protocols to minimize the risk of any additional COVID-19 exposures. This included having clinical research coordinators work remotely on the informed consent process with patients over the phone or through videoconferencing prior to a healthcare professional obtaining the final signature, reducing the need for extra people in patient rooms.
“We now have an electronic regulatory system that has improved the speed and efficiency of our consent and compliance processes while reducing some of the need for in-person interactions that have to be as limited as possible during a pandemic,” comments Dr. Thomas. He believes this system and other new practices and innovations that began as pandemic adaptations will help MCW grow clinical research moving forward.
“After initially consolidating to only the most essential trials, as the pandemic went on and policies changed, we developed an evaluation process for determining which additional trials could resume or begin at each stage of recovery,” says Dr. Thomas. This reactivation process was completed when the current recovery phase began in May 2021, under which all clinical research projects can proceed with team members following MCW and clinical location guidelines for preventing COVID-19 transmission.
“While COVID-19 certainly affected our plans and pace of growth for a time, it also has revealed the heroic character of everyone who has stepped up to keep clinical research open as a unique resource for our community,” notes Dr. Thomas. “We truly are one of the pivotal clinical research entities in the Midwest, and we plan to keep growing and improving so we can provide more patients with the best and most innovative care.”
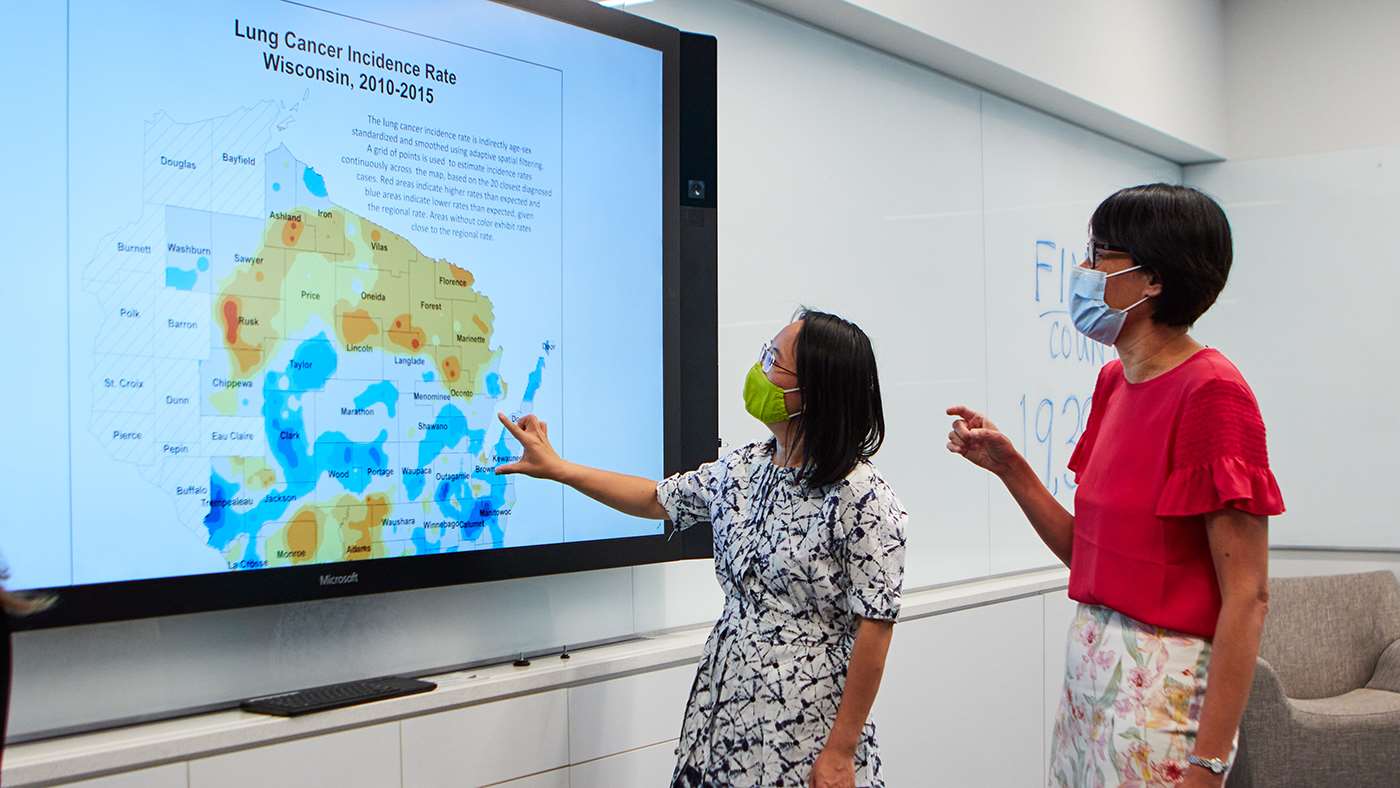
Scientists Engaging with Communities
Community-engaged researchers at MCW initially focused on listening to their partners at a wide variety of government agencies and nonprofit organizations to understand how priorities were changing in response to the COVID-19 pandemic.
“Early on, many of our partners significantly shifted their focus to basic needs, so we rolled up our sleeves and joined them,” says Staci Young, PhD, MCW’s interim director for community engagement, interim senior associate dean for community engagement, associate professor of family and community medicine and director of the Center for Healthy Communities and Research. “We worked on providing food, diapers and other necessities, including figuring out how to produce facial coverings at scale and distribute them en masse to organizations and community members.”
Dr. Young notes that a core value of community-engaged research is to be nimble and responsive to changing needs, and that the COVID-19 pandemic crystallized the importance of this tenet. One area of need Dr. Young quickly discovered was related to Wisconsin’s safety net clinics.
“Some clinics were concerned about closing, as they didn’t have the resources to provide telehealth services,” adds Dr. Young. She garnered funding from the Advancing a Healthier Wisconsin Endowment at MCW to work with the Wisconsin Association of Free and Charitable Clinics to develop a telehealth platform that would work for safety net clinics and their patients.
“For me, this was about access to care,” remarks Dr. Young. “Without these safety net clinics, the only alternative for many patients would be to turn to already overburdened hospital emergency rooms.” Dr. Young and her team worked with telehealth vendor Updox to build a statewide telehealth network supported by enhanced infrastructure and training at the 41 participating free and charitable clinics. These clinics had delivered more than 4,300 telehealth patient visits by May 2021.
As Dr. Young and her MCW colleagues continue to work with community partners on regional and statewide priorities related to the COVID-19 pandemic, she also is looking ahead to what the future may bring for MCW’s community-engaged researchers. Fortunately, she sees some hopeful signs.
“There are more scholarly activities and grant proposals going out as of late. Some community events are happening with the appropriate safety precautions, which has been very encouraging to see. And there is definitely an increase in momentum for moving both longstanding and newer work forward with our partners,” says Dr. Young.
Growing to Accelerate Discovery
Fortunately, because of the resilience and adaptability of MCW scientists, the challenges of the COVID-19 pandemic have not altered the institution’s trajectory of growth as a center for research in Wisconsin and beyond. The total amount of funding garnered by MCW scientists over five years from federal, private industry and other external sources has increased by nearly 50 percent, from $158.2 million to $235.9 million. This includes an institutional record of $122.6 million from the National Institutes of Health (NIH) in funding that MCW researchers earned for federal fiscal year 2020.
In addition, MCW ranks in the top third of all US medical schools for NIH research support. This increasing support helps equip MCW investigators with the resources needed to be pioneers in their fields of expertise, including using the latest technical innovations to accelerate the process of discovery. This growth also is reflected in MCW’s clinical research portfolio. MCW has the largest clinical research enterprise in Wisconsin and has more than doubled its clinical trial revenue since 2014.
“I’m not surprised we’ve been this successful,” says Dr. Hillard. “We have the right ingredients in terms of the talent of our research faculty members and our collaborative culture. Our people really drive team science forward, which delivers the best results.”

Enhancing Research Infrastructure
To continue to support and propel growth in its research mission, MCW is renovating and preparing for the construction of new facilities that will provide the necessary infrastructure for tomorrow’s discoveries. The renovation of the 45-year-old Basic Science Building (BSB) began in September 2018. In addition to modernizing the BSB, a major goal is to develop an open laboratory environment that encourages and facilitates better connectivity among labs to increase collaboration and interdisciplinary discovery. In June 2019, renovations on the second floor were completed. By July 2019, the department of microbiology & immunology moved into its new home in the BSB and became the first department to occupy renovated space. Construction on the fourth floor also is complete, and renovations are in progress on the fifth floor. Work on the sixth floor will follow once the fifth floor is completed.
In addition to renovating the BSB, MCW continues planning for construction of a new cancer research building on its Milwaukee campus. This investment reflects MCW’s long-term commitment to leading cancer research and treatment for the people of Wisconsin – a priority that has garnered support from the state. In 2019, Wisconsin Governor Tony Evers and the State Legislature committed a $10 million State Building Commission grant within the 2019-2021 biennial state budget toward a cancer research building for MCW. When complete, the building will be Milwaukee’s only research facility dedicated to cancer and will support scientists and physicians advancing research that addresses the unique cancer burden in southeastern Wisconsin and beyond with the ultimate goal of improving clinical outcomes for all patients.
MCW has selected CannonDesign as the design partner for the cancer research building. The firm’s award-winning team of planners, advisors, architects and specialized laboratory designers has worked with numerous top-tier cancer centers designated by the National Cancer Institute. The design phase for the building began in fall 2021 and will continue into 2022.
“By investing in our research infrastructure and talent, MCW is accelerating the translation of research discoveries into improved patient care, resulting in better health outcomes for our communities,” says Joseph E. Kerschner, MD ’90, FEL ’98, provost and executive vice president, and the Julia A. Uihlein, MA, Dean of the MCW School of Medicine.
Research Cores and Shared Services
Research cores and shared services are important components of MCW’s collaborative research ecosystem. Whether the focus is on sharing blisteringly fast supercomputers or structural biochemistry expertise, grouping specialized technology and knowledge into designated research cores and shared services provides greater access to these resources for labs and clinical investigators throughout MCW. Currently, MCW offers dozens of research cores and shared services in areas ranging from bioinformatics to cellular physiology, imaging and protein chemistry.
School of Medicine Research Strategic Plan
The 2025 School of Medicine Research Strategic Plan was approved by the MCW Board of Trustees in June 2019 to provide a focused effort on growing the research enterprise.
This decision was reached following extensive work by the research planning team and external experts to synthesize qualitative information from interviews and focus groups with leaders, partners and scientists, as well as quantitative analysis of MCW’s research enterprise and national strategic funding priorities.
At that time, a $300 million academic quasi-endowment was established so that its earnings could help support the growth of the research enterprise guided by the Research Strategic Plan.
To facilitate the implementation of the plan, faculty and staff workgroups were established to gather additional data and generate recommendations for investment and programmatic improvements related to the plan’s focus areas of research culture, data science, neurosciences, cardiovascular disease and cancer.
– Greg Calhoun



