
Aron Geurts, PhD
John E. and Genevieve M. Butenhoff Cardiovascular Research Professorship
Locations
- Physiology
Contact Information
Honors and Awards
Research Experience
- Animals, Genetically Modified
- Blood Pressure
- Disease Models, Animal
- Genetic Engineering
- Genetic Research
- Genomics
- Hypertension
- Kidney
- Organisms, Genetically Modified
- Phenotype
- Rats
- Rats, Inbred Dahl
Methodologies and Techniques
- Animals, Genetically Modified
- Cloning, Molecular
- CRISPR-Cas Systems
- Gene Editing
- Gene Expression Profiling
- Gene Knockout Techniques
- Genetic Techniques
- Genetic Vectors
- Genomics
- Models, Genetic
- Mutation
- Organisms, Genetically Modified
MCW Program / Core Facilities
- Rodent Model Resource Center (Director)
Research Interests
Continuing research efforts in the Geurts lab are being driven by our interests in developing genetic approaches toward understanding human health and disease. For the past 12 years, we have been developing tools for genetic manipulation in a variety of cell and animal systems including stem cells, zebrafish, mice and laboratory rats. These systems are among the most widely preferred models for genetic and physiological investigation into human disease, however, genetic approaches, especially in non-mouse systems, have traditionally been limited by a lack of technologies.
After joining the Medical College in 2006, we implemented new approaches to accelerate transgenic and gene knockout studies for the PhysGen Program for Genomic Applications by adapting the Sleeping Beauty transposable element system for use in rats. Transposons are currently the most reproducible and efficient tool available for adding new genes to the rat genome and since then, we have worked with several other local investigators to create new transgenic rat models.
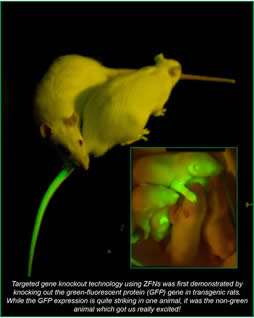
More recently, the Geurts lab has been developing TAL Effector Nuclease (TALEN) technology for targeted genome engineering. TALENs are a relatively new technology which are analogous to ZFNs, but have some attractive attributes including reduced cost and design flexibility which will facilitate their use in the field. This new technique is complemented by our recent development of the first rat embryonic stem cells from a hypertensive rat model in collaboration with the laboratory of Dr. Howard Jacob. The availability of stem cells from this disease model rat now provides unique possibilities for creating more complicated genetic models. We are currently establishing whether these cells are capable of supporting our engineering approaches for producing genetically modified rats.
Recently, Dr. Geurts' creative and innovative contributions to the field of genetics and technology were recognized by the granting of a New Innovator Award from the Office of the Director of the National Institutes of Health. This prominent award will propel efforts in the Geurts lab toward pushing the limits of these technologies to create better models of human disease. These techniques, animal models, and resources broadly benefit the local and broader research communities and advance our collective understanding of complex human genetic diseases.
Publications
-
Monitoring biological effects of somatic cell genome editing.
(Freedman BS, Bulte JWM, Conklin BR, Judge LM, Dwinell MR, Geurts AM, Sitton MJ, Mahajan V, Kiani S, Gersbach CA, Ebrahimkhani MR, Kelly JJ, Ronald JA, Morizane R, Gupta N, Shakeri-Zadeh A, Vo N, Saha K, Saxena S, Gamm DM, Sinha D, Tarantal AF, Vandsburger M, Matsubara A, Fu H, Tsai SQ, SCGE Dissemination and Coordinating Center Toolkit Team, SCGE Biological Effects Initiative.) Nat Rev Genet. 2026 Jan 13 PMID: 41530266 SCOPUS ID: 2-s2.0-105027880322 01/14/2026
-
(Martínez-Beamonte R, Guillén N, Sánchez-Marco J, Herrera-Marcos LV, Surra JC, Navarro MA, Barranquero C, Arnal C, Puente JJ, Rodríguez-Yoldi MJ, Mendiara I, Domeño C, Nerín C, Geurts AM, Osada J, Laclaustra M.) Int J Mol Sci. 2025 Dec 12;26(24) PMID: 41465421 PMCID: PMC12732954 SCOPUS ID: 2-s2.0-105025961107 12/30/2025
-
(Yang C, Dave DD, Boovarahan SR, Shimada S, Geurts A, Dash RK, Cowley AW Jr.) Function (Oxf). 2025 Oct 28;6(6) PMID: 41055389 PMCID: PMC12560232 SCOPUS ID: 2-s2.0-105020316608 10/07/2025
-
The role of carnitine palmitoyl transferase 2 in the progression of salt-sensitive hypertension.
(Dissanayake LV, Smith BA, Zietara A, Levchenko V, Lowe M, Kravtsova O, Shapiro A, Upadhyay G, Halade GV, Geurts AM, Palygin O, Staruschenko A.) Am J Physiol Cell Physiol. 2025 Oct 01;329(4):C1188-C1202 PMID: 40897451 PMCID: PMC12462776 SCOPUS ID: 2-s2.0-105017327500 09/03/2025
-
(Takizawa A, Foeckler J, Knapp E, Grzybowski M, Geurts AM, Carroll J, Merriman DK.) Mol Reprod Dev. 2025 Sep;92(9):e70055 PMID: 40891659 PMCID: PMC12826629 SCOPUS ID: 2-s2.0-105014800254 09/02/2025
-
Aberrant recursive splicing in a human disease locus.
(Boone PM, Harripaul R, Yadav R, Grzybowski M, Hanafy MK, Lee AC, Choi EY, Collins RL, Polesskaya O, Makhortova N, Larson MO, Kayir H, Wang Y, Avila RA, Frie JA, Eed A, Albeely AM, Venmuri S, Ayoub SM, Lemanski JM, Ben-Isvy D, Zhao X, Sanchis-Juan A, Handley M, Erdin S, de Esch C, Mohajeri K, Chen C, Tovar PG, Salani M, Oliveira MM, Tai DJC, Currall B, McGraw C, Slaughenhaupt S, Doan R, Gao D, Gusella JF, Sanchez-Roige S, Young J, Khokar J, Geurts AM, Palmer AA, Talkowski ME.) bioRxiv. 2025 Aug 15 PMID: 40919386 PMCID: PMC12413155 09/08/2025
-
(Liu Y, Pandey R, Qiu Q, Liu P, Xue H, Wang J, Therani B, Ying R, Usa K, Grzybowski M, Yang C, Mishra MK, Greene AS, Cowley AW Jr, Rao S, Geurts AM, Widlansky ME, Liang M.) Nat Commun. 2025 Jul 17;16(1):6577 PMID: 40675959 PMCID: PMC12271514 SCOPUS ID: 2-s2.0-105011067559 07/18/2025
-
Physiological role and mechanisms of action for a long noncoding haplotype region.
(Xue H, Mishra MK, Liu Y, Liu P, Grzybowski M, Pandey R, Usa K, Vanden Avond MA, Bala N, Alli AA, Cowley AW Jr, Qiu Q, Greene AS, Rao S, O'Meara CC, Geurts AM, Liang M.) Cell Rep. 2025 Jun 24;44(6):115805 PMID: 40493452 PMCID: PMC12263090 SCOPUS ID: 2-s2.0-105007437771 06/10/2025
-
A Novel GH Deficient Rat Model Reveals Cross-Species Insights Into Aging.
(Myint SMMP, Lasher AT, Liu K, Geurts AM, Austad SN, Sun LY.) Aging Cell. 2025 Jun 05;24(8):e70126 PMID: 40474491 PMCID: PMC12341780 SCOPUS ID: 2-s2.0-105007351623 06/06/2025
-
(Fitzpatrick MK, Dyson C, Beeson A, Adrian L, Marrs G, Grzybowski M, Klotz J, Geurts AM, Chen R, Weiner JL, Solberg Woods LC.) Genes Brain Behav. 2025 Jun;24(3):e70028 PMID: 40492272 PMCID: PMC12149762 SCOPUS ID: 2-s2.0-105007863205 06/10/2025
-
(Martínez-Beamonte R, Guillén N, Sánchez-Marco J, Herrera-Marcos LV, Surra JC, Navarro MA, Barranquero C, Arnal C, Puente JJ, Rodríguez-Yoldi MJ, Mendiara I, Domeño C, Nerín C, Geurts AM, Osada J, Laclaustra M.) International Journal of Molecular Sciences. December 2025;26(24) SCOPUS ID: 2-s2.0-105025961107 12/01/2025
-
(Fitzpatrick MK, Dyson C, Beeson A, Adrian L, Marrs G, Grzybowski M, Klotz J, Geurts AM, Chen R, Weiner JL, Solberg Woods LC.) Genes Brain and Behavior. June 2025;24(3) SCOPUS ID: 2-s2.0-105007863205 06/01/2025


