
Neil Hogg, PhD
Professor; Senior Associate Dean of Academic Affairs, School of Graduate Studies; Vice Chair for Education in Biophysics; Program Director, Biophysics Graduate Program; Director, Redox Biology Program
Locations
- Biophysics
MFRC 2039
Contact Information
Education
PhD, Biological Chemistry, University of Essex, Colchester, England, 1993
MSc, Molecular Biochemistry, University of Essex, Colchester, England, 1989
BSc (honors), Biochemistry, University of Leeds, Leeds, England, 1987
Biography
I received my BSc (honors) in biochemistry at University of Leeds in the United Kingdom, and my MSc in molecular biochemistry and PhD in biological chemistry from University of Essex, United Kingdom. I completed my postdoctoral training in free radical biology in the MCW Department of Biophysics. I was appointed as MCW faculty in 1996.

Research Experience
- Anemia, Sickle Cell
- Nitric Oxide
- Nitric Oxide Donors
- Nitric Oxide Synthase
- Oxidative Stress
- Peroxynitrous Acid
- Reactive Nitrogen Species
- S-Nitroso-N-Acetylpenicillamine
- S-Nitrosoglutathione
Methodologies and Techniques
- Electron Spin Resonance Spectroscopy
- Nitric Oxide
- Reactive Nitrogen Species
Leadership Positions
- Associate Dean of Academic Affairs, Graduate School of Biomedical Sciences
- Director, Redox Biology Program
MCW Program / Core Facilities
- National Biomedical EPR Center
Research Interests
Nitric Oxide Signaling
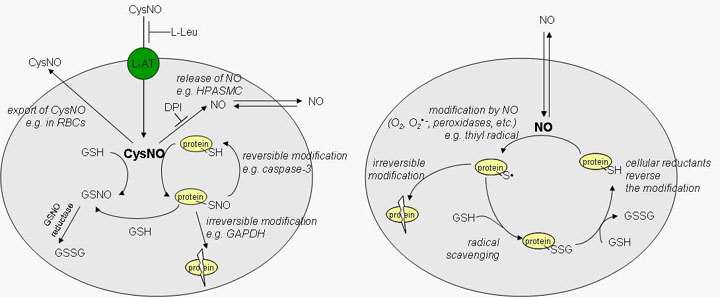
Nitric oxide (NO) is a free radical that is synthesized in biology by the nitric oxide synthase (NOS) family of enzymes. Though a plethora of literature describes the many, sometimes contradictory, effects of NO, much less known about the mechanisms by which these effects occur. Some molecular targets have been firmly established (such as the binding of NO to heme groups in guanylyl cyclase), whereas other potential targets for NO are less clearly defined. One major area of research in this laboratory is to elucidate the mechanisms by which NO and metabolites of NO interact with and modify protein targets to generate intracellular signals that can affect cellular function and potentially differential gene expression. We are conducting experiments to examine how NO transmits signals in cells using both global proteomic approaches and more directed approaches aimed at candidate proteins. Our ultimate goal is to define the mechanisms by which NO affects cellular functions at the level of individual protein modifications.
Sickle Cell Disease
Sickle cell disease (SCD) is one of the most known genetic diseases, and it has been established for many years that it is caused by a single amino acid mutation in the gene for the beta chain of hemoglobin. Despite its fame and prevalence (particularly in Africa and in populations of African descent), there is a pressing need for an increased understanding of this disease and for novel effective therapeutic approaches. SCD is characterized by hemoglobin polymerization in response to deoxygenation that results in distorted and rigid red blood cells, which may occlude vessels, leading to many of the features of this disease. However, it has become clear that simple blood vessel occlusion cannot explain many of the symptoms, and researchers are looking beyond the sickled RBC for clues to how this pathology progresses and how it can potentially be treated. New data relate many aspects of SCD pathology to intravascular hemolysis and an increase in plasma hemoglobin. This can interfere with normal nitric oxide-dependent vascular function and also potentially act as a pro-oxidant, leading to vascular dysfunction and altered endothelial adhesiveness. In collaboration with Drs. Cheryl Hillery and Nancy Wandersee in the Department of Pediatrics, we are conducting experiments to examine the role of intravascular hemolysis in SCD and in another hemolytic anemia, hereditary spherocytosis.
Nitrite as a Source of Nitric Oxide
While nitric oxide is mainly generated from L-arginine by nitric oxide synthase (NOS) enzymes, there is a growing realization that additional NO-generating pathways exist. The most common idea is that nitrite can be reduced back to NO via the nitrite reductase activity of some proteins and enzymes. While this is generally an activity that is associated with prokaryotes, several mammalian proteins and enzymes have currently been reported to have a low level nitrite reductase activity. These include xanthine oxidoreductase, cytochrome c oxidase, and, strangely enough, NOS itself. Interestingly, deoxygenated hemoglobin also possesses nitrite reductase activity (a fact realized as long ago as 1945), and it has recently been demonstrated that this activity represents a mechanism by which plasma nitrite can be converted back to vasoactive NO under deoxygenated conditions and so represents a hypoxically activated vasodilatory mechanism. We are currently studying the mechanisms of nitrite reduction and the role this plays in vascular pathology.
Past Postdoctoral Fellows
Agnes Keszler
Past Graduate Students
Netanya Spencer (2003)
Yanhong Zhang (2004)
Kasia Broniowska (2008)
Nick Kettenhoffen (2009)
Timothy Flewelen (2013)
Zhen Ding (2013)
Ching-Fang Chang (2014)
Lab Members
Agnes Keszler, Research Scientist I
Publications
-
(Rarick KR, Li K, Teng RJ, Jing X, Martin DP, Xu H, Jones DW, Hogg N, Hillery CA, Garcia G, Day BW, Naylor S, Pritchard KA Jr.) Free Radic Biol Med. 2025 Jun;233:419-420 PMID: 40148177 SCOPUS ID: 2-s2.0-105001160156 03/28/2025
-
β-Cell-selective regulation of gene expression by nitric oxide.
(Naatz A, Yeo CT, Hogg N, Corbett JA.) Am J Physiol Regul Integr Comp Physiol. 2024 Jun 01;326(6):R552-R566 PMID: 38586887 PMCID: PMC11381020 SCOPUS ID: 2-s2.0-85195535629 04/08/2024
-
Sterile inflammation induces vasculopathy and chronic lung injury in murine sickle cell disease.
(Rarick KR, Li K, Teng RJ, Jing X, Martin DP, Xu H, Jones DW, Hogg N, Hillery CA, Garcia G, Day BW, Naylor S, Pritchard KA Jr.) Free Radic Biol Med. 2024 Mar;215:112-126 PMID: 38336101 PMCID: PMC11290318 SCOPUS ID: 2-s2.0-85187405718 02/10/2024
-
(Teng RJ, Jing X, Martin DP, Hogg N, Haefke A, Konduri GG, Day BW, Naylor S, Pritchard KA Jr.) Free Radic Biol Med. 2021 Apr;166:73-89 PMID: 33607217 PMCID: PMC8009865 SCOPUS ID: 2-s2.0-85101585619 02/20/2021
-
(Larson MC, Hogg N, Hillery CA.) Int J Mol Sci. 2021 Jan 27;22(3) PMID: 33513958 PMCID: PMC7865243 SCOPUS ID: 2-s2.0-85099909407 01/31/2021
-
Nitric Oxide Circumvents Virus-Mediated Metabolic Regulation during Human Cytomegalovirus Infection.
(Mokry RL, Schumacher ML, Hogg N, Terhune SS.) mBio. 2020 Dec 15;11(6) PMID: 33323506 PMCID: PMC7773989 SCOPUS ID: 2-s2.0-85098068781 12/17/2020
-
(Quesnelle K, Guimaraes DA, Rao K, Singh AB, Wang Y, Hogg N, Shiva S.) Nitric Oxide. 2020 Nov 01;104-105:36-43 PMID: 32891753 PMCID: PMC7606822 SCOPUS ID: 2-s2.0-85090420429 09/07/2020
-
(Stancill JS, Happ JT, Broniowska KA, Hogg N, Corbett JA.) Am J Physiol Regul Integr Comp Physiol. 2020 May 01;318(5):R1004-R1013 PMID: 32292063 PMCID: PMC7272767 SCOPUS ID: 2-s2.0-85084379321 04/16/2020
-
Nitric oxide circumvents virus-mediated metabolic regulation during human cytomegalovirus infection
(Mokry RL, Schumacher ML, Hogg N, Terhune SS.) Mbio. November-December 2020;11(6):1-25 SCOPUS ID: 2-s2.0-85098068781 11/01/2020
-
The Role of Metabolic Flexibility in the Regulation of the DNA Damage Response by Nitric Oxide.
(Oleson BJ, Broniowska KA, Yeo CT, Flancher M, Naatz A, Hogg N, Tarakanova VL, Corbett JA.) Mol Cell Biol. 2019 Sep 15;39(18) PMID: 31235477 PMCID: PMC6712938 SCOPUS ID: 2-s2.0-85071713105 06/27/2019
-
(Keszler A, Lindemer B, Weihrauch D, Jones D, Hogg N, Lohr NL.) Free Radic Biol Med. 2019 Feb 01;131:443 PMID: 30581102 SCOPUS ID: 2-s2.0-85058702220 12/26/2018
-
Ascorbate attenuates red light mediated vasodilation: Potential role of S-nitrosothiols.
(Keszler A, Lindemer B, Hogg N, Lohr NL.) Redox Biol. 2019 Jan;20:13-18 PMID: 30261342 PMCID: PMC6156744 SCOPUS ID: 2-s2.0-85053754114 09/28/2018

