John E. Baker, PhD
Professor Baker's research program serves as a nexus to translate basic science discoveries into clinical applications at the Medical College of Wisconsin and Children's Wisconsin. Dr. Baker has developed and maintained strong collaborations between basic scientists and clinicians to translate laboratory findings made in his Medical College of Wisconsin-based space into clinical applications.
He has achieved this through extensive interactions and collaborations with basic scientists and clinicians. Dr. Baker is professor of surgery, biochemistry, and pharmacology and toxicology at the Medical College of Wisconsin. He was awarded a PhD in biochemistry from the University of London in 1984.
Thrombopoietin receptor agonists protect human heart cells against injury
Protection of the heart against injury from acute ischemia remains challenging for emergency physicians and cardiologists because there are no therapies proven to directly protect the heart against the deleterious effects of ischemia in humans. Despite major advances in the care of patients with acute coronary syndrome over many decades, rates of early morbidity and mortality associated with the condition remain unacceptably high.
Dr. Baker’s research has shown Eltrombopag and thrombopoietin confer immediate protection to human cardiac myocytes against injury from hypoxia/reoxygenation by decreasing necrotic and apoptotic cell death in a concentration-dependent manner, with an optimal concentration of 3 µM for eltrombopag and 1.0 ng/ml for thrombopoietin (J Pharm Exp Ther 2015; 352, 429-437). The extent of protection conferred with eltrombopag is equivalent to that of thrombopoietin. This is the first description of the protective actions of eltrombopag and thrombopoietin on human cardiac myocytes.
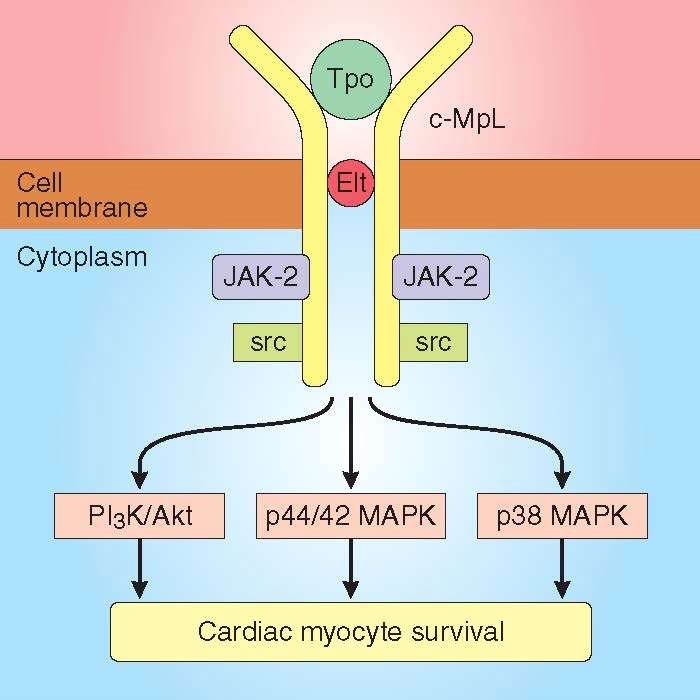
Eltrombopag and thrombopoietin activate multiple prosurvival pathways. Inhibition of Janus kinase-2, proto oncogene tyrosine-protein kinase, protein kinase B/phosphatidylinositol-3 kinase, p44/42 mitogen-activated protein kinase, and p38 mitogen-activated protein kinase abolished cardiac myocyte protection by eltrombopag and thrombopoietin. All pathways appear important for cardioprotection in that inhibition of any one pathway is sufficient to block the cardioprotective effect. The results from this study support the presence of a functional thrombopoietin receptor in human cardiac myocytes that is activated upon binding of a thrombopoietin receptor agonist to its receptor to increase myocyte survival during hypoxia.
These findings are significant to cardioprotection because of their potential to improve human outcomes from acute ischemic events including myocardial infarction. The discovery that thrombopoietin confers protection to organs in the context of acute ischemic events provides solid rationale to minimize the extent of damage post-event. When administered intravenously under novel pharmacokinetic parameters, thrombopoietin confers highly significant protective properties against cellular damage arising from ischemia/reperfusion events. Furthermore, thrombopoietin confers cardioprotection without affecting platelet production at a dose around 20 times lower than currently used clinically. The potential for clinical application is high. Eltrombopag is FDA approved and in clinical use. In addition to its ability to protect heart, thrombopoietin protects rat brain against injury from stroke.