ReyLab Research & Publications
Utilizing electrodes implanted in patients suffering from epilepsy and used to monitor brain activity, ReyLab performs cutting-edge research built on three major themes: understanding human episodic memory, improving epilepsy diagnosis and treatment, and developing new tools for data acquisition and analysis of electrophysiological data in the human brain.
Understanding the Building Blocks of Human Episodic Memory
 About
About
Studies in animals have demonstrated that the medial temporal lobe (MTL) plays a key role in declarative memory. These findings have also been supported by numerous studies involving patients with lesions in the MTL, who show impairment accessing and combining contextual information and recalling details from past experiences or imagining hypothetical situations. It has been postulated that neurons in the human medial temporal lobe hold the meaning of a stimulus for episodic memory functions, and that each conceptual entity is encoded in an assembly of “concept cells” that, when activated, brings the specific concept into awareness. This requires sparse multimodal invariant representations, as we tend to remember relationships between concepts and disregard irrelevant details. Memories would then be created by associating different events through the coactivation of concept cell assemblies. At ReyLab, we exploit the opportunity to record from individual neurons of the human brain while subjects perform cognitive tasks. We will continue investigating concept cells as we seek to understand what information drives them, which will be accomplished by dynamically exploring the stimulus space that elicits the response of a given neuron, and we will seek to understand how concept cells interact with downstream cortical areas to encode and recall episodic memories.
Image
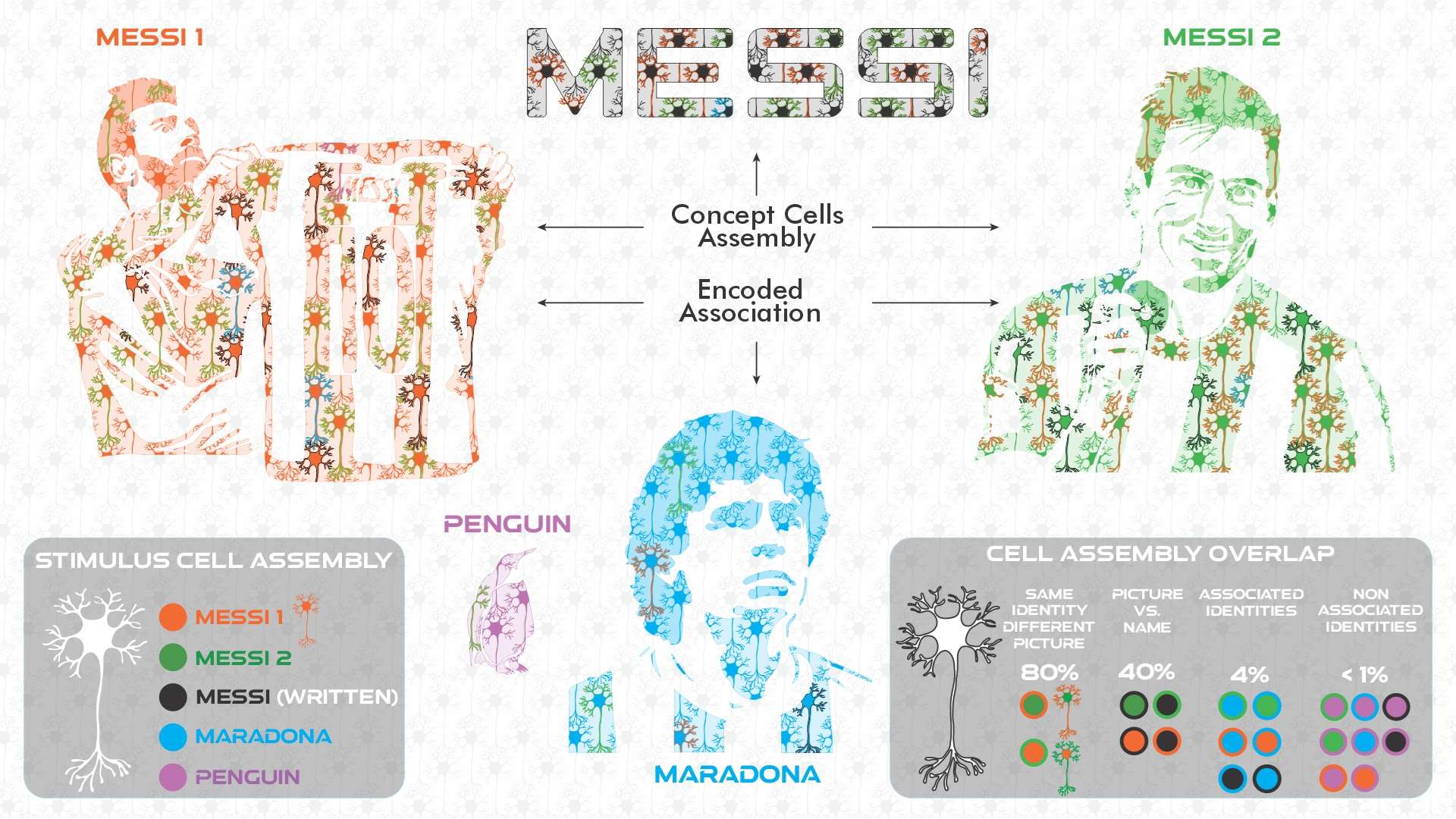
Illustration summarizing how concept cell assemblies and associations are encoded. When an image of football star Messi ("MESSI 1") is perceived by someone, a cell assembly of medial temporal lobe (MTL) neurons will be activated in response to the stimulus (all the neurons in orange in the illustration). When another image of Messi is presented ("MESSI 2"), a different cell assembly will be engaged (neurons in green), but with a large overlap (~80%) with the previous assembly (the neurons in such overlap are depicted with two colors for fill and contour; in this case orange/green or green/orange), so that any of those stimuli will lead to a response from the neurons in the overlap. When the written name is presented, we get yet another assembly (neurons in black), but the overlap with the previous assemblies will be smaller (~40%), as the very different sensory information carried by the name leads to very distinct cortical inputs to the MTL. In addition, when an image of Maradona is presented (a highly associated concept to Messi, with them being the two best players in the history of football), another assembly will be engaged (neurons in blue), and with a fairly small overlap with the Messi assemblies (~4%). However, such overlap is much larger to the one we would see with other non-associated concepts (<1%), like a penguin (neurons in purple). These partially overlapping representations could be the neural basis for encoding and learning concepts, associations, and ultimately, episodic memories. Illustrated by @darwid_illustration.
Representative Publications
Simultaneous Multiscale Recordings of the Brain (from single neurons to EEG) for Improved Epilepsy Diagnosis and Treatment
 About
About
Epilepsy is one of the most common neurological disorders, affecting almost 1% of the worldwide population and over 3.5M people in the US alone. Epilepsy commonly leads to reduced quality of life, morbidity, and death. In approximately 30% of patients, antiepileptic drugs fail to control seizures. Epilepsy not controlled by medication is known as refractory epilepsy. In some cases, non-invasive methods might not be sufficient to identify the area of the brain that is necessary for the appearance of seizures, also known as the epileptic zone (EZ). In these cases, intracranial recordings (iEEG) may be captured with use of electrodes implanted into the brain. In addition to the implanted macro (clinical) electrodes, microwires can be inserted in the lumen of the probe, allowing us to record single-neuron activity and local field potentials (LFP), which account for mean activation across several neurons. By extracting information at different scales (single neuron, LFP, iEEG), ReyLab will be looking to identify better biomarkers and improve the definition of the EZ. This better definition will aid in resection, improving seizure-free rate and reducing consequent functional deficits, and inform better treatment options, such as responsive neuro-stimulation.
Image
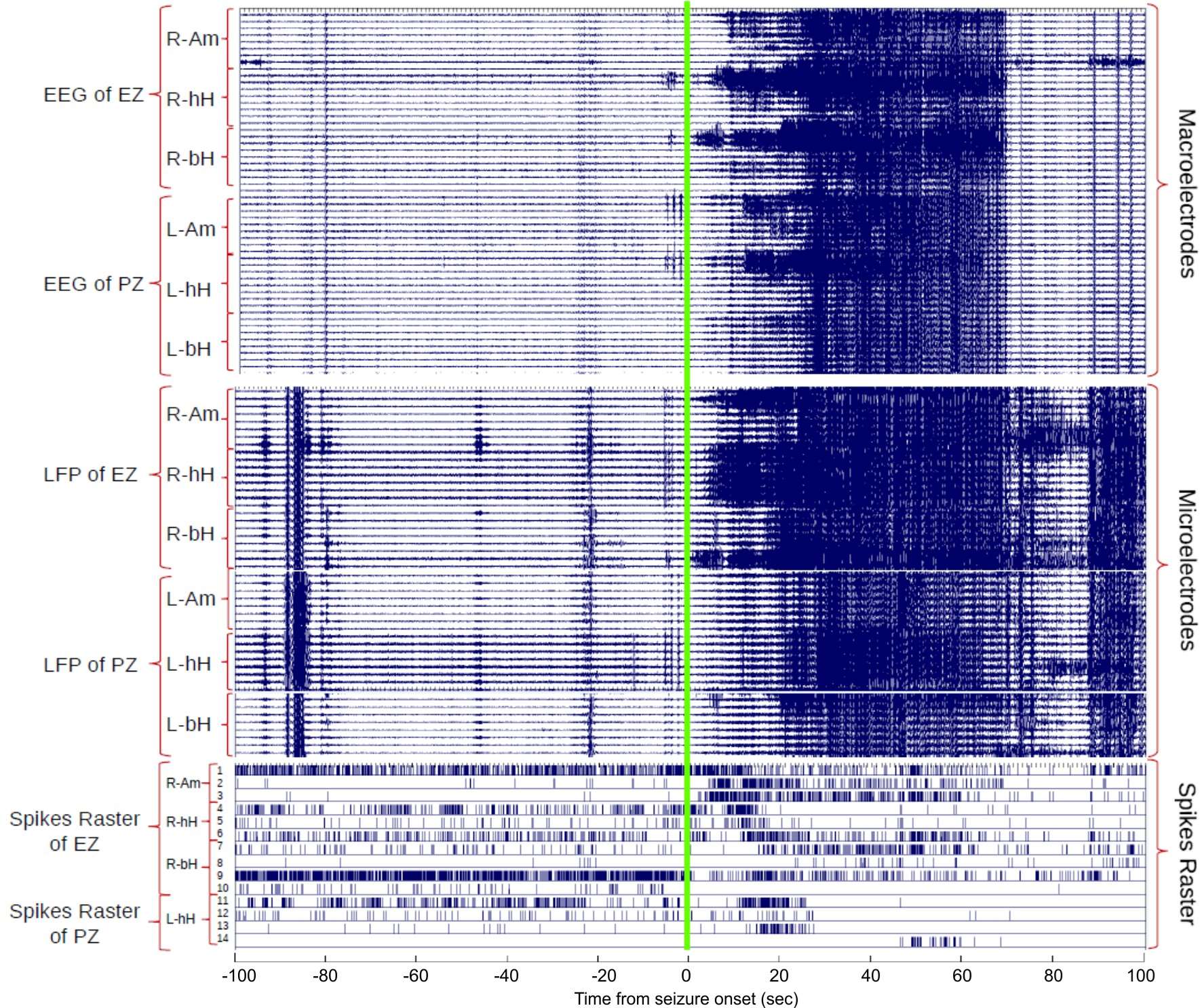
Spontaneous seizure recorded at different scales in the brain. Although iEEG (macroelectrodes) and LFP (microelectrodes) show similar behavior in the raw data displayed, which is driven mainly by low frequency activity, a large heterogeneity can be seen in the activity of different putative neurons in the epileptogenic and propagation zones (EZ and PZ, respectively).
Development of New Tools for Data Acquisition and Analysis of Electrophysiological Data in the Human Brain
About
Exploiting all the capabilities of state-of-the-art electrophysiology recording devices requires integration of output from technologies created by different manufacturers. Employing this abstraction layer makes it possible to operate different devices and develop improved generic acquisition tools. For example, ReyLab developed a graphical user interface to monitor and characterize ongoing recordings (online) and a framework to run online, dynamic cognitive tasks, including spike sorting of microwire recordings. In addition, ReyLab is interested in developing offline tools for data analysis, as other research themes studied in the lab require customized analysis tools to accommodate specific needs. For example, ReyLab is developing a new spike sorting development pipeline to tackle the challenges associated with microwire recordings in humans, including tracking of neurons throughout long-term recordings. Moreover, to support epilepsy research, ReyLab develops methods to automatically detect and classify different biomarkers in LFPs and iEEGs, including interictal epileptiform discharges and high-frequency oscillations. Finally, ReyLab actively collaborates in open software projects that follow standards designed to improve analytic methods and the accessibility, reliability, and reproducibility of data collected.
Images
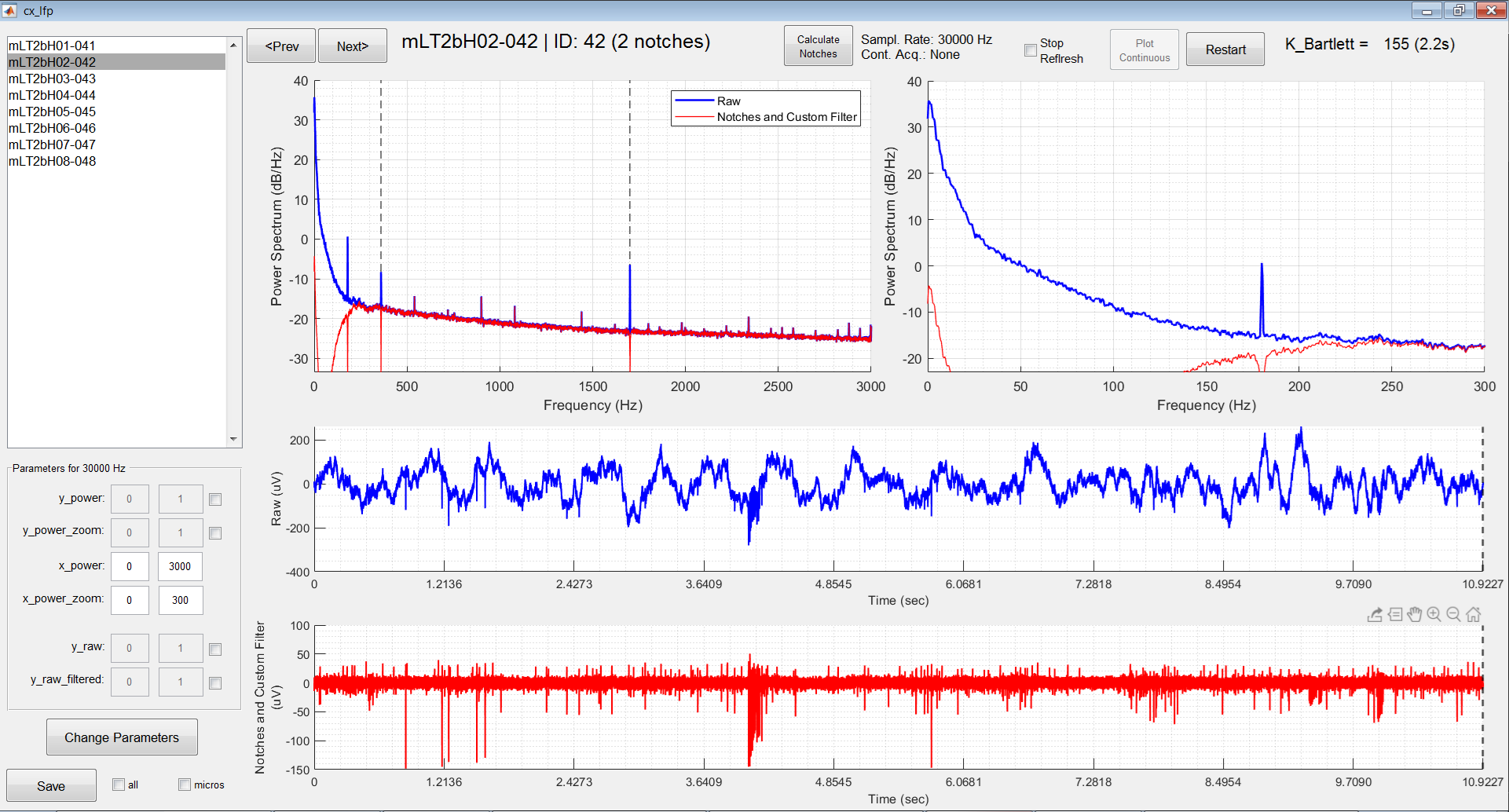
Top: Neural recording captured online through the GUI “cx_lfp” developed by ReyLab. The time series can be observed raw (blue) and bandpass filtered (red), but the frequency response is also averaged across several windows (~2s long each) so we can have a good estimate of the power spectrum through the periodogram. Spiking activity can be clearly seen in the filtered time series.
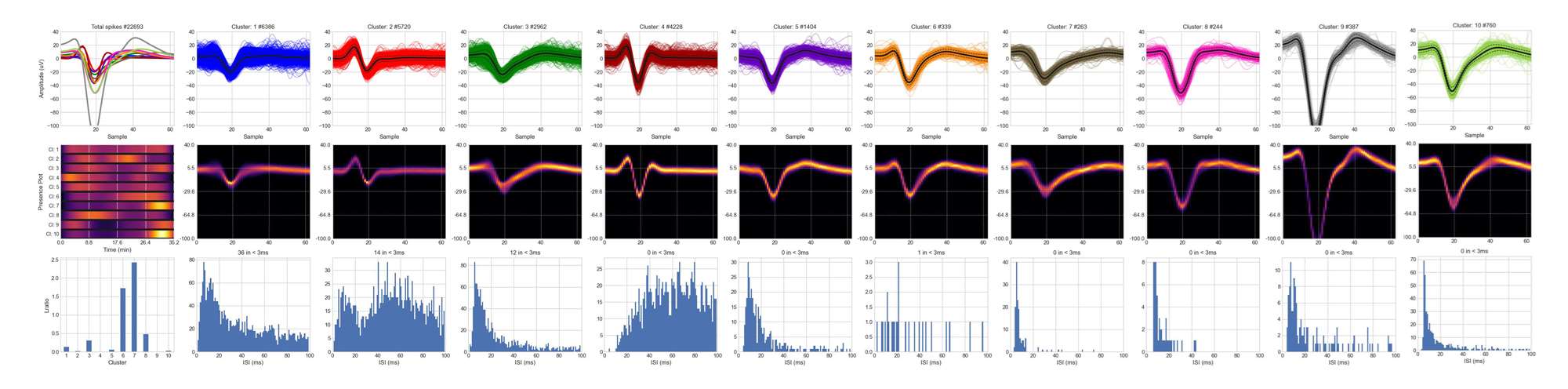
Bottom: When spike sorting was applied to the same channel (using a pipeline also developed by ReyLab), we were able to isolate 10 different putative single neurons. For each of them, we can see the overlapped waveforms, the 2D-probability density function estimate, and the interspike interval (ISI) histograms.
Representative Publications
Recent Publications
-
(Kang JU, Mattar L, Vergara J, Gobo VE, Rey HG, Heilbronner SR, Watrous AJ, Hayden BY, Sheth SA, Bartoli E.) Neuroimage. 2025 Jul 15;315:121288 PMID: 40409386 SCOPUS ID: 2-s2.0-105005851016 05/24/2025
-
Lack of context modulation in human single neuron responses in the medial temporal lobe.
(Rey HG, Panagiotaropoulos TI, Gutierrez L, Chaure FJ, Nasimbera A, Cordisco S, Nishida F, Valentin A, Alarcon G, Richardson MP, Kochen S, Quian Quiroga R.) Cell Rep. 2025 Jan 28;44(1):115218 PMID: 39823228 PMCID: PMC11781864 SCOPUS ID: 2-s2.0-85214797893 01/17/2025
-
Intracranial directed connectivity links subregions of the prefrontal cortex to major depression
(Myers J, Xiao J, Mathura RK, Shofty B, Gates V, Adkinson J, Allawala AB, Anand A, Gadot R, Najera R, Rey HG, Mathew SJ, Bijanki K, Banks G, Watrous A, Bartoli E, Heilbronner SR, Provenza N, Goodman WK, Pouratian N, Hayden BY, Sheth SA.) Nature Communications. December 2025;16(1) SCOPUS ID: 2-s2.0-105010187958 12/01/2025
-
Feature-based encoding of face identity by single neurons in the human amygdala and hippocampus
(Cao R, Wang J, Lin C, De Falco E, Peter A, Rey HG, Brunner P, Willie JT, DiCarlo JJ, Todorov A, Rutishauser U, Li X, Brandmeir NJ, Wang S.) Nature Human Behaviour. 2025 SCOPUS ID: 2-s2.0-105007328811 01/01/2025

