Chaurasia Lab Research Overview

Research Projects
DAMPs in Diabetic Eye Diseases
Diabetic eye diseases (DEDs) are the leading cause of vision impairment worldwide. DRDs include disorders like diabetic keratopathy (DK), diabetic retinopathy (DR), and diabetic macular edema (DME). Long-term diabetes can damage the eyes, increasing the risk of cataracts and glaucoma, which may impair vision or lead to blindness. The OIAL team is developing a preclinical model for DK (PMID: 35002212) and DR (PMID: 29847637) using an Ossabaw minipig fed a Western diet depicting metabolic syndrome (MetS). The lab is currently exploring the function of damage-associated molecular pattern (DAMP) proteins, which are endogenous molecules released during ischemic/cellular stress that serve as danger signals and activate the innate immune system in DEDs.

Team Members: Utkarsh R. Addi
Clinical Partners: Baseer Ahmad, MD, Thomas Connor, MD, Aparna Ramasubramanian, MD, Edward M. Barnett, Md, PhD, Judy Hoggatt, MD
Collaborators: Veluchamy A. Barathi, Rajiv R. Mohan, Arkasubhra Ghosh, Mike, Mouhamad
Funding: ARVO Foundation for Eye Research, NIH National Eye Institute
Small Leucine-Rich Proteoglycans in Retinopathy of Prematurity
Retinal ischemia is a key pathological condition associated with various retinal disorders, including age-related macular degeneration (AMD), retinal vein occlusion (RVO), diabetic retinopathy (DR), and retinopathy of prematurity (ROP). Understanding the structural and cellular components essential to the pathophysiology of retinal diseases is critically important. The OIAL team at MCW has identified small leucine-rich proteoglycans (SLRPs), such as Decorin (Dcn) and Biglycan (Bgn), which are integral components of the extracellular matrix (ECM) in connective tissues. They play a vital role in cell-matrix interactions and the signaling pathways involved in cell migration, oxygen availability, permeability, fibrillogenesis, and angiogenesis, which are crucial for maintaining structural and vascular homeostasis in the retina (PMID: 34298915). We conducted a cross-sectional study to examine the association between aqueous humor (AH) decorin levels, if any, and the severity of DR (PMID: 34947953). Decorin concentrations are significantly associated with visual acuity (LogMAR) measurements and the severity of DR due to a compensatory response to the retinal microvascular changes during hyperglycemia. Our recent work in ROP identifies its critical role in retinal ischemia, particularly in maintaining structural and vascular integrity in the retina.

Team Members: Shermaine Low, Waylon Alvarado, Anju Thomas, Yesenia Gomez, Steven Trinh
Clinical Partners: Deborah Costakos, MD, MS, Aparna Ramasubramanian, MD, Baseer Ahmad, MD, Tom Connor, MD
Collaborators: Rajiv R. Mohan, Arkasubhara Ghosh
Funding: CRI22708, NIH R01 submitted
Ocular Manifestations of Fabry Disease
Fabry disease is an X-linked lysosomal storage disorder that affects multiple systems, including renal, cardiovascular, and neurological systems, as well as the eyes. It is caused by a gene mutation in the lysosomal exoglycohydrolase, α-galactosidase A (α-Gal A or GLA), leading to an inborn error of glycosphingolipid metabolism. Fabry is the most common lysosomal storage disease, with a prevalence of 1 in 1,237 to 1 in 1,334. No curative treatments are available for Fabry disease. An α-Gal A KO rat model was established at MCW, depicting several systemic pathologies in cardiac, renal, and neurological tissues. Most importantly, our recent studies depict an ocular phenotype of Fabry disease similar to those seen in human patients. We conducted a comprehensive study on ocular abnormalities in the anterior segment of a rat model of Fabry disease, examining age (young, adult, and aged), sex (male and female), and genotype (wild-type, knockout male, heterozygous female, and knockout female) using multimodal clinical imaging techniques.

Team Members: Sanjay Ch, Amer Mohiuddin, Steven Trinh
Clinical Partners: Vinicius De Stefano, MD, PhD
Collaborators: Iris Kaseem, MD, PhD
Funding: NIH R01EY030077
Eye Cancers
Uveal melanoma (UM) is a rare form of intraocular malignancy derived from extracutaneous melanocytes residing in the uveal tract of the eye. UM accounts for 5% of all melanomas, of which 90% of cases arise in the choroid, 6% in the ciliary body, and 4% in the iris. In the United States, UM prevalence is estimated at approximately five cases per million individuals. Although common genetic and environmental risk factors have been recognized, the causative agents' precise nature is unknown. Current treatment modalities are directed towards tumor destruction, preventing recurrence and metastasis, and conserving vision. Irradiation therapies (plaque brachytherapy and photon radiotherapy) and surgical enucleation excisions remain the most therapeutic approaches for uveal melanoma. However, there are several ophthalmologic complications, including radiation retinopathy, cataracts, and glaucoma, that affect long-term vision. Despite its rarity, uveal melanoma has gathered the utmost attention due to its aggressive behavior, with a propensity for metastasis to secondary sites, including the liver, lung, bone, and skin.
Topotecan is a topoisomerase 1 inhibitor that creates double-stranded breaks in DNA, thereby causing cell death. Topotecan is a chemotherapy drug used singly or in combination with other medications for the management of cervical, ovarian, and small-cell lung cancer. In ocular cancers, topotecan has been administered intravenously in children with relapsed/refractory metastatic and intraocular retinoblastoma. Currently, clinical trials are underway for the delivery of topotecan using an episcleral delivery system (chemoplaque) in retinoblastoma. Preliminary results from these clinical trials demonstrate sustained tumor response in retinoblastoma with no systemic detectable topotecan. However, topotecan and its application in uveal melanoma remain relatively unexplored. In 2012, a single in vitro study investigated topotecan's effect on uveal melanoma cells. Considering the lack of scientific evidence for its doses and efficacy in vitro, further validation is essential to understand topotecan's therapeutic potential and underlying mechanisms in uveal melanoma.
Team Members: Anju Thomas, Audrianna Wu
Clinical Partners: Aparna Ramasubramanian, MD
Collaborators: Arkasubhra Ghosh, Narayana Nethralaya, Bangalore, India
Funding: A Cure for Sight Foundation
Fibrotic Eye Diseases
Cellular transdifferentiation of stromal fibroblasts to myofibroblasts is a critical rate-limiting step in corneal wound healing. These active and contractile cells are essential for effective wound closure through extracellular matrix (ECM) deposition and stromal collagen reorganization after injury. They express α-smooth muscle actin (αSMA) and are preceded by precursor cells that express vimentin and desmin. Myofibroblasts typically undergo apoptosis post-injury, allowing infiltrating keratocytes to close the wound. However, excessive accumulation of myofibroblasts and irregular ECM secretion leads to aberrant wound healing, which may result in vision loss, clinically recognized as corneal haze. Current treatment strategies include off-label Mitomycin C (MMC) use, which is controversial due to dosage and long-term efficacy. Some reports suggest MMC is safe and effective, but its long-term use can cause wound healing dysfunction. Steroidal treatments may raise intraocular pressure and cause cataracts. Surgical options like phototherapeutic keratectomy (PTK) are limited to superficial scarring. Hence, there's a need for a safe and effective drug for corneal fibrosis. The OIAL is interested in exploring new therapeutic targets regulating multiple signaling pathways (TGF/Smad/BMP7 and IL10/SOCS), such as inhibition of differentiation (Id) proteins, redefining the wound healing mechanisms.
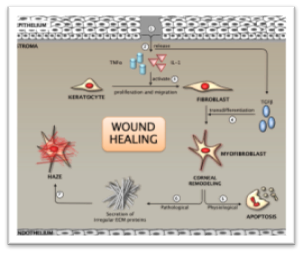
Clinical Partners:
Collaborators: Rajiv R. Mohan, University of Missouri, Columbia, MO
Funding: NIH R01EY030774
S100 Proteins in Diabetic Retinopathy
The S100 family of small Ca2+-binding proteins is involved in many inflammatory diseases, but their role in the retina remains unknown. In 2019, the team discovered S100A9, a myeloid-related protein, associated with the severity of DR (PMID: 31336525) in patients with Type 2 diabetes (T2D). The team is currently investigating the functional role of S100A9 in diabetic retinopathy. We recently determined that S100A9 is primarily secreted by microglial cells in response to diabetic stress, which results in sterile inflammation by activating the NLRP3 inflammasome signaling pathway (PMID 39554084). The present study will evaluate the functional role of S100A9 using S100A9Tg (gain-of-function) and S100A9-/- (loss-of-function) studies in the retina and explore its possibility of being a novel VEGF-independent molecular target in DR pathogenesis.

Team Members: Scholastica Go, Utkarsh Reddy, Anju Thomas
Clinical Partners: Tom Connor, MD, Aparna Ramasubramanian, MD, Baseer Ahmad, MD
Funding: NIH R01EY029795
Decorin in the Retinal Vascular Development
Decorin is a small leucine-rich proteoglycan (SLRP) that regulates the extracellular matrix and angiogenesis in many tissues. However, its function in the retina remains unknown. Our pilot studies with loss-of-function studies (Dcn-/- and Dcn-/+ mice) suggest a critical role for decorin in retinal microvasculature. Our preliminary data suggest that mice lacking decorin (Dcn) exhibit aberrant vascular sprouting in the deep and intermediate plexus on postnatal day 7 (P7), emphasizing the significance of complex Dcn matrix scaffold-neurogliovascular unit crosstalk during the early stages of vascular development in the retina (Figure 1). Matrix assembly/disassembly is a complex process involving a variety of proteins, including SLRPs (i.e., Dcn), and exploring their role in retinal vascular development is an unmet need, making a solid premise to study the novel role of Dcn in vascular ECM remodeling and matrix assembly neuronal and glial regulators. The present investigation aims to decipher its therapeutic potential during physiological retinal vascular development.

Clinical Partners: Deborah Costakos, MD, MS, Aparna Ramasubramanian, MD, Baseer Ahmad, MD, Tom Connor, MD
Funding: AHW LOI submitted in 2024
NIH CounterACT Program for Ocular Fibrosis and Angiogenesis
Sulfur mustard (SM), an alkylating agent, is used as a chemical weapon in World Wars and Syria. SM exposure severely affects the eye and other organs, causing ocular abnormalities in up to 90% of those exposed. Acute injury leads to severe pain, corneal issues, photophobia, chemosis, and blindness, while chronic effects include keratopathy and retinal dysfunction. Currently, no comprehensive animal study details early or late SM effects on retinal damage. However, a human study found a significant reduction in retinal function (b-wave ERG) in Iranian veterans exposed to SM, indicating defects in retinal layers and Müller glial cells, resulting in neural degeneration. Preliminary rabbit data suggested mustard gas damage and gliosis in Müller glia. A major knowledge gap exists regarding SM's role in retinal toxicity. Our studies hypothesized that SM causes biphasic retinal damage: short-term glial hyperactivation and long-term neurodegenerative dysfunction due to defective mitophagy. We will test these using Göttingen minipig eyes exposed to SM and perform retinal glial studies with commercial human Müller glial cells and pig microglia culture models.

Team Members: Utkarsh Reddy Addi, Sanjay Ch, Yesenia Gomez
Clinical Partners: Baseer Ahmad, MD, Tom Connor, MD, Aparna Ramasubramanian, MD, Judy Hoggatt, MD
Collaborators: Rajiv R. Mohan
Funding: NIH NIAID CounterACT Grant R56EY035223; U01EY031650
Cardiometabolic Syndrome
Diabetes mellitus (DM) causes chronic hyperglycemia, leaving patients at high risk for heart failure despite tight glycemic control. Diabetes-induced heart failure (DHF) creates metabolic challenges through altered cardiac substrate metabolism, increased fatty acid oxidation, decreased glucose utilization, and impaired mitochondrial function. These changes induce oxidative stress, lipotoxicity, and energy deficits, worsening heart failure. Research has uncovered mechanisms of metabolic remodeling in diabetic hearts, including autophagy dysregulation, epigenetic changes, polyamine regulation, and branched-chain amino acid (BCAA) metabolism. Such factors aggravate mitochondrial dysfunction and metabolic inflexibility, hindering cardiac function. Therapies to enhance glucose oxidation, modify fatty acid metabolism, and optimize ketone body use show potential for restoring metabolic balance and improving heart health. This review discusses molecular mechanisms of metabolic remodeling in diabetic hearts, highlights new methodologies, and outlines recent therapeutic strategies to slow DHF progression. These insights offer opportunities for targeted therapies addressing the metabolic roots of heart failure in diabetes.

Clinical Partners: Mark Carlson
Collaborators: Paras Kumar Mishra, University of Nebraska Medical Center, Omaha, Nebraska
Funding: NIH R01 Submitted in 2024
Ocular Immunology
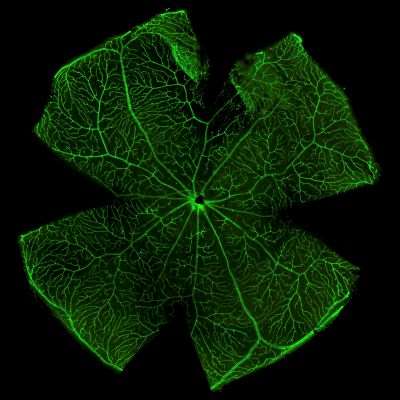
Isolectin Staining
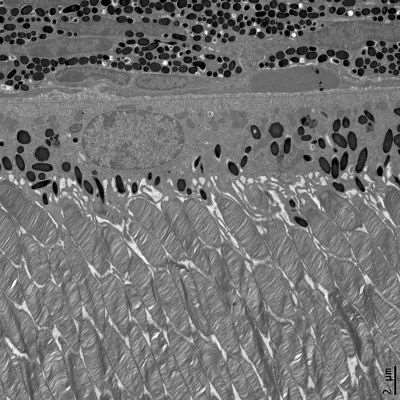
Photoreceptors and RPE

