Pepsin and Reflux Laboratory
To contact Dr. Johnson, please email njohnston@mcw.edu.
Learn More about Dr. Johnston





Development of Pepsin Inhibitors as a Novel Therapeutic Approach for Reflux Disease: Recent and Ongoing Research
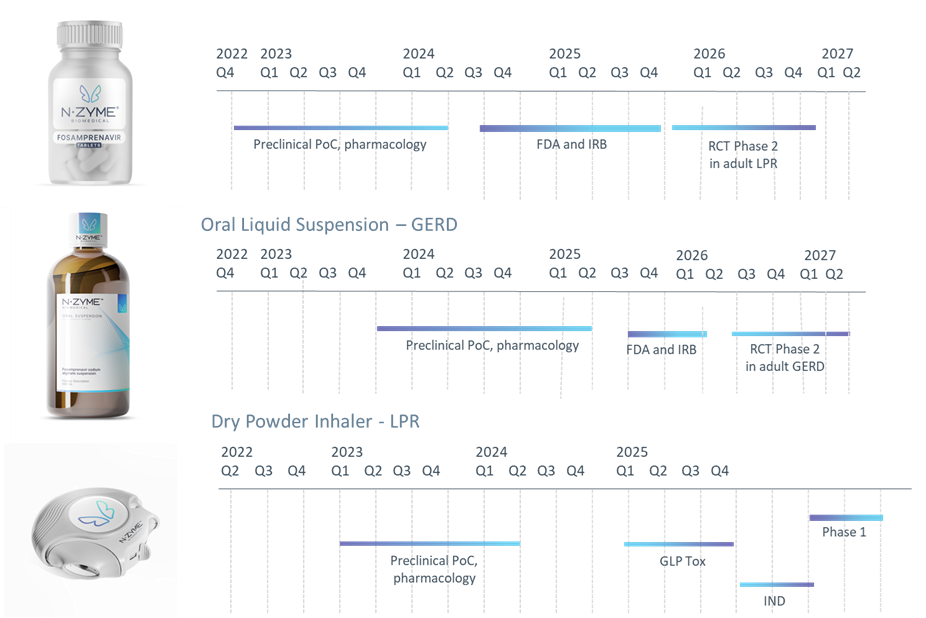
Oral fosamprenavir for the treatment of laryngopharyngeal reflux (LPR)
Preclinical Studies
Laboratory-based investigation of the efficacy and cell and molecular protective effects of fosamprenavir and its prodrug, amprenavir, in models of the human aerodigestive tract.
In vivo efficacy study of fosamprenavir in an LPR mouse model
- Johnston N, Samuels TL, Goetz CJ, Arnold LA, Smith BC, Seabloom D, Wuertz B, Ondrey F, Wiedmann TS, Vuksanovic N, Silvaggi NR, MacKinnon AC, Miller J, Bock J, Blumin JH. Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model. Laryngoscope. 2023 Jan;133 Suppl 1(Suppl 1):S1-S11. doi: 10.1002/lary.30242. Epub 2022 Jun 9. PMID: 35678265; PMCID: PMC9732152.
Protection against epithelial barrier disruption and downstream cancer-associated effects of weakly acidified pepsin in an in vitro model
- Samuels TL, Blaine-Sauer S, Yan K, Johnston N. Amprenavir inhibits pepsin-mediated laryngeal epithelial disruption and E-cadherin cleavage in vitro. Laryngoscope Investig Otolaryngol. 2023 Jun 22;8(4):953-962. doi: 10.1002/lio2.1102. PMID: 37621274; PMCID: PMC10446255.
Clinical Trials
Evaluation of a repurposing strategy, using FDA-approved HIV-1 protease inhibitor, fosamprenavir (commercial tablets) for symptoms of LPR.
- Phase II Proof of Concept Study: A 12-Week Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Assess the Efficacy of Oral Fosamprenavir for Laryngopharyngeal Reflux.
Oral fosamprenavir sodium alginate for PPI refractory gastroesophageal reflux disease (GERD)
Preclinical Studies
Protection against epithelial barrier disruption and downstream cancer-associated effects of weakly acidified pepsin encountered during proton pump inhibitor (PPI) recalcitrant GERD
- Blaine-Sauer S, Samuels TL, Yan K, Johnston N. The Protease Inhibitor Amprenavir Protects against Pepsin-Induced Esophageal Epithelial Barrier Disruption and Cancer-Associated Changes. Int J Mol Sci. 2023 Apr 5;24(7):6765. doi: 10.3390/ijms24076765. PMID: 37047737; PMCID: PMC10095080.
Protection against global transcriptomic changes caused by weakly acidified pepsin mimicking PPI-recalcitrant GERD
- Ergun P, Samuels TL, Mathison AJ, Liu T, Jin VX, Johnston N. Amprenavir Mitigates Pepsin-Induced Transcriptomic Changes in Normal and Precancerous Esophageal Cells. Int J Mol Sci. 2025 Jun 26;26(13):6182. doi: 10.3390/ijms26136182. PMID: 40649959; PMCID: PMC12250232.
Esophageal mucoadhesion and conversion of a novel fosamprenavir-sodium alginate formulation in an ex vivo porcine model (study underway)
Clinical Trial
Evaluation of the efficacy of a novel fosamprenavir-sodium alginate formulation designed for superior esophageal mucoadhesion and local esophageal delivery.
- Phase II Proof of Concept Study: Randomized, Double-blind, Placebo-Controlled
Trial of Fosamprenavir-Sodium Alginate Administered Orally for 8 Weeks to Patients with Proton Pump Inhibitor Refractory Gastroesophageal Reflux Disease. Estimated Start: Sept 2026
Fosamprenavir Dry Powder Inhaler for LPR
Preclinical Studies
Laboratory-based investigation of cell and molecular protective effects of fosamprenavir and its prodrug, amprenavir, in models of the human aerodigestive tract.
In vivo efficacy study of inhaled fosamprenavir in an LPR mouse model
- Johnston N, Samuels TL, Goetz CJ, Arnold LA, Smith BC, Seabloom D, Wuertz B, Ondrey F, Wiedmann TS, Vuksanovic N, Silvaggi NR, MacKinnon AC, Miller J, Bock J, Blumin JH. Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model. Laryngoscope. 2023 Jan;133 Suppl 1(Suppl 1):S1-S11. doi: 10.1002/lary.30242. Epub 2022 Jun 9. PMID: 35678265; PMCID: PMC9732152.
Pre-GLP safety testing of inhaled fosamprenavir and design of a laryngopharyngeal dry powder inhaler
- Lesnick A, Samuels TL, Seabloom D, Wuertz B, Ojha A, Seelig D, Ondrey F, Wiedmann TS, Hogan C, Torii E, Ouyang H, Yan K, Garcia GJM, Bock JM, Johnston N. Inhaled fosamprenavir for laryngopharyngeal reflux: Toxicology and fluid dynamics modeling. Laryngoscope Investig Otolaryngol. 2024 Jan 24;9(1):e1219. doi: 10.1002/lio2.1219. PMID: 38362183; PMCID: PMC10866582.
- Dey S, Jadcherla A, Johnston N, Bock JM, Garcia GJM. Optimizing dry powder inhaler for laryngopharyngeal reflux: Effects of particle size and breathing technique. Journal of Aerosol Science 2025 189, 106641.
GLP toxicologic assessment (to be completed late 2025)
Clinical Trials
Evaluation of the safety and efficacy a novel inhaled fosamprenavir formulation that offers targeted laryngopharyngeal delivery for reduced dosing.
- Phase I Safety/Efficacy Study
Drug Development Timeline
Informational Videos
New Approach to Reflux Treatment Could Revolutionize Care for LPR and GERD Header
Dr. Johnston’s research has uncovered a crucial culprit in reflux disease: pepsin, a digestive enzyme produced in the stomach.
European Union of Phonetricians Congress Presentation 2023
Presenter: Tina Samuels, PhD
Taiwan Voice Society Annual Meeting in conjunction with International Voice Symposium of TCVGH September 2022
Presenter: Nikki Johnston, PhD
Learn more about Dr. Johnston and her team's research accomplishments.
Current Laboratory and Study Team Members

Jonathan Bock, MD
Professor

Joel H. Blumin, MD
Chief, Professor

Pelin Ergun, PhD
Postdoctoral Researcher

Nikki Johnston, PhD
Professor, Otolaryngology & Communication Sciences and Microbiology & Immunology

Ally Lesnick
Research Coordinator

Karolina Lungova
Medical Student

Tina Samuels, MS
Program Manager, Airway, Digestive, & Voice Research

Mark A. Vukovich, MSN, APNP
Nurse Practitioner
Recent Publications
-
(Ergun P, Samuels TL, Mathison AJ, Liu T, Jin VX, Johnston N.) Int J Mol Sci. 2025 Jun 26;26(13) PMID: 40649959 PMCID: PMC12250232 SCOPUS ID: 2-s2.0-105010318417 07/13/2025
-
Unveiling the Silent Struggle: A Comprehensive Guide to Laryngopharyngeal Reflux Disease.
(Husain IA, Johnston N.) Otolaryngol Clin North Am. 2025 Jun;58(3):xv-xvi PMID: 40185652 SCOPUS ID: 2-s2.0-105001827920 04/05/2025
-
Pepsin, Mucosal Injury, and Pathophysiology of Non-acid Reflux.
(Samuels TL, Johnston N.) Otolaryngol Clin North Am. 2025 Jun;58(3):415-432 PMID: 40148170 SCOPUS ID: 2-s2.0-105001138800 03/28/2025
-
(Dey S, Jadcherla A, Johnston N, Bock JM, Garcia GJM.) Journal of Aerosol Science. September 2025;189 SCOPUS ID: 2-s2.0-105009433292 09/01/2025
-
(Ergun P, Samuels TL, Mathison AJ, Liu T, Jin VX, Johnston N.) International Journal of Molecular Sciences. July 2025;26(13) SCOPUS ID: 2-s2.0-105010318417 07/01/2025


