Nelson Lab Research

Research Overview
The Nelson Lab focuses on characterizing and improving patient outcomes for traumatic brain injury, or TBI. Using a variety of quantitative—and sometimes qualitative— methods, the Nelson Lab investigates a diverse ranges of TBI-related problems, posing questions such as:
- What is the typical course of TBI recovery from the immediate post-injury period onward, such as the areas of subjective symptoms, observable clinical signs, cognitive performance, and day-to-day functioning?
- What are the subtypes of TBI that might inform more precision medicine approaches to treatment?
- How and why do patients differ in their TBI outcomes?
- How can measures of TBI-related disability be made more sensitive to better reflect treatment effects in clinical trials?
- What improvements to clinical care would patients with TBI and their families value?
- How can healthcare systems better adopt evidence-based practices and improve care pathways for persons with TBI?
Active Research Projects
Active research projects in the Nelson Lab include phenotyping traumatic brain injury, improving outcome measurement for clinical trials, understanding individual differences in TBI recovery and outcomes, as well as developing patient-centered systems for care for TBI.
Phenotyping Traumatic Brain Injury
TBI is a heterogeneous injury that affects patients in diverse ways. The Nelson Lab uses latent variable modeling strategies such as factor analysis and latent profile analysis with large, multicenter samples of athletes and community members with TBI to understand heterogeneity in how patients present after TBI, with goals of identifying subtypes of injury that may inform more personalized assessment and treatment approaches.
Representative Publications
- Modeling the structure of acute sport-related concussion symptoms: A bifactor approach
- Latent profile analysis of neuropsychiatric symptoms and cognitive function of adults 2 weeks after traumatic brain injury: Findings from the TRACK-TBI study
- Neurobehavioral traits as transdiagnostic predictors of clinical problems
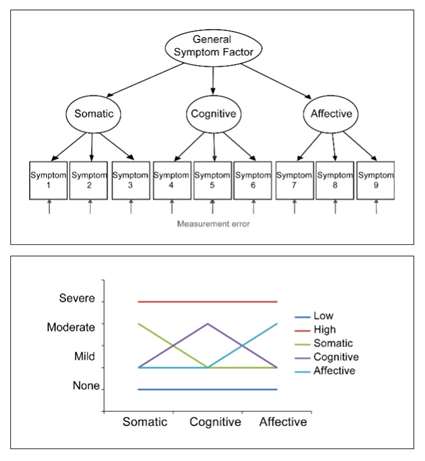
Images
Top: Example of a factor model showing how one can conceptualize and estimate latent dimensions of Somatic, Cognitive, and Affective symptoms by modeling the correlations among 9 observed symptom ratings. The dimensions of Somatic, Cognitive, and Affective symptoms are subsumed by a common factor that explains their correlations.
Bottom: A hypothetical latent class model showing how a sample who has rated themselves on Somatic, Cognitive, and Affective symptom dimensions could be stratified into 5 qualitatively distinct subgroups, who differ in their symptom profile. In this example the symptom profiles are Low symptoms, High symptoms, and Somatic, Cognitive, or Affective symptoms only.
Improving Outcome Measurements for Clinical Trials
Expert's believe the field's long history of unsuccessful clinical trials is, in part, a result of its use of an outcome measure that is not sufficiently sensitive to individual differences in TBI-related disability and recovery. Investigators at the Nelson Lab use Item Response Theory (IRT) and other psychometric tools to understand and improve clinical outcome measures, such as measures of TBI-related symptoms, functional limitations, quality of life, and cognitive performance. Goals of this work are to produce clinical trial endpoints that are better able to detect treatment effects; more accurately reflect patients' experiences of TBI; and improve evidence-based personalized outcome measurement strategies for clinical and translational studies of TBI.
Representative Publications
- Validating multidimensional outcome assessment using the TBI Common Data Elements: An analysis of the TRACK-TBI Pilot sample
- Validity of the Brief Test of Adult Cognition by Telephone (BTACT) in Level 1 trauma center patients 6 months post traumatic brain injury: A TRACK-TBI study
- Functional Status Examination yields higher measurement precision than the Glasgow Outcome Scale-Extended after moderate-to-severe traumatic brain injury
- Improving the precision of the Glasgow Outcome Scale-Extended using item response theory: A TRACK-TBI study
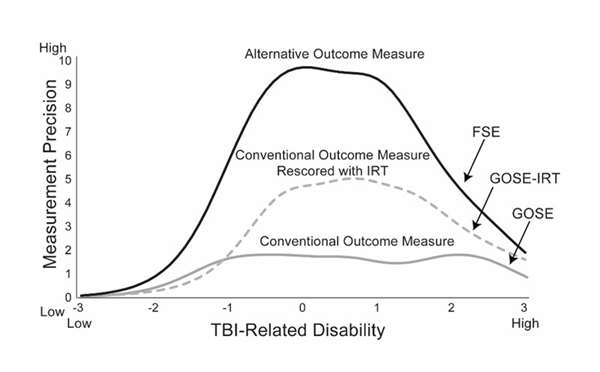
Image
Figure compares the measurement precision of 3 traumatic brain injury disability outcome measures. The x-axis reflects estimated disability level, as modeled by an item response theory model. The y-axis displays the test information of each of the 3 outcome measures. The measure with the lowest information is the Glasgow Outcome Scale Extended (GOSE), a conventional outcome measure treated as an ordinal 8-level variable. The measure with the next highest information is the GOSE rescored using item response theory. The measure with the highest information is the Functional Status Examination, another measure of injury-related disability.
Understanding Individual Differences in Traumatic Brain Injury Recovery and Outcomes
 Improving personalized medicine for TBI will require better understanding why individuals have such different outcomes from what appear to be similar injuries. We investigate how diverse neurobiological, psychosocial, and environmental factors influence acute presentation and outcome from TBI, which will inform ongoing efforts to build systems of care for TBI that address our patients' needs.
Improving personalized medicine for TBI will require better understanding why individuals have such different outcomes from what appear to be similar injuries. We investigate how diverse neurobiological, psychosocial, and environmental factors influence acute presentation and outcome from TBI, which will inform ongoing efforts to build systems of care for TBI that address our patients' needs.
Representative Publications
- Acute clinical predictors of symptom recovery in emergency department patients with mild traumatic brain injury and non-traumatic brain injuries
- Recovery following mild traumatic brain injury in patients presenting to U.S. Level 1 trauma centers: A TRACK-TBI study
- Relationship between neighborhood disadvantage and mild traumatic brain injury symptoms
- Functional recovery, symptoms, and quality of life 1–5 years after traumatic brain injury: A TRACK-TBI study
Image
Figure displays a circular puzzle with 4 quadrants. The top left quadrant conveys pre-injury risk and resilience factors such as genetics, neurophysiology, social determinants, cognitive reserve, psychiatric symptoms, and personality traits. The bottom left quadrant conveys injury characteristics such as cause of injury, TBI characteristics, polytrauma, and complications. The bottom right quadrant conveys environment, exemplified by treatment and rehabilitation, social support, financial resources, secondary gain, and repeated trauma exposure. The top left quadrant conveys injury response, exemplified by symptoms, attributions/expectancies, cognitive sequelae, neurophysiologic response, and epigenetics.
 Developing Patient-Centered Systems for Care for Traumatic Brain Injury
Developing Patient-Centered Systems for Care for Traumatic Brain Injury
There is a great need for the development of standardized, coordinated systems of care for persons with TBI around the globe. The Nelson Lab's ongoing projects—led by a multi-stakeholder group of persons with TBI, clinicians, hospital administrators, researchers, and others—is performing mixed-methods research, quality improvement initiatives, and implementation science studies to build better healthcare systems for community members with TBI.
Image
Figure displays a pie chart with 3 segments—patient feedback, stakeholder engagement, and clinical improvements. Arrows linking the segments convey that they inform each other.
Recent Publications
-
(Eagle SR, Lamb B, Huber D, McCrea MA, Tarima S, deRoon-Cassini TA, Okonkwo DO, Nelson LD.) Clin Neurol Neurosurg. 2025 Sep;256:109017 PMID: 40532286 PMCID: PMC12291057 SCOPUS ID: 2-s2.0-105008205601 06/19/2025
-
(Temkin N, Barber J, Machamer J, Sugar G, Morrissey MR, Boase K, Zahniser E, Bodien YG, Giacino JT, McCrea MA, Nelson LD, Stein MB, Taylor S, Robertson C, Okonkwo D, Manley G, Dikmen S, TRACK-TBI Investigators.) J Neurotrauma. 2025 Sep;42(17-18):1550-1559 PMID: 40200868 SCOPUS ID: 2-s2.0-105003882256 04/09/2025
-
Vascular Risk Factors and 1-Year Cognitive Change Among Individuals With Traumatic Brain Injury.
(Schneider ALC, Hunzinger KJ, Brett BL, Sandsmark DK, Jain S, Sun X, Gardner RC, Manley GT, Diaz-Arrastia R, Nelson LD, TRACK-TBI Study Investigators, Belton P, Eagle S, Gopinath S, Grandhi R, Keene CD, Krishnamoorthy V, Mac Donald C, McCrea M, Merchant R, Mukherjee P, Ngwenya LB, Okonkwo D, Robertson C, Schnyer D, Taylor SR, Yue JK, Zafonte R.) JAMA Netw Open. 2025 Aug 01;8(8):e2525719 PMID: 40779268 PMCID: PMC12334961 SCOPUS ID: 2-s2.0-105013233906 08/08/2025
-
(Nelson LD, Gray S, Barry CO, Young SA, TBI System of Care Collaborators.) Mayo Clin Proc Innov Qual Outcomes. 2025 Aug;9(4):100630 PMID: 40584562 PMCID: PMC12205785 07/01/2025
-
(Thomas DG, Erpenbach H, Smith CN, Hickey RW, Waltzman D, Haarbauer-Krupa J, Nelson LD, Patterson CG, McCrea M, Collins MW, Kontos AP.) J Pediatr. 2025 Aug;283:114596 PMID: 40254050 SCOPUS ID: 2-s2.0-105004917264 04/21/2025
-
(Simons MU, Maio A, Huber DL, Corrigan JD, Temkin N, Darsie M, Kitagawa R, Whyte J, Giacino JT, Stein MB, Manley GT, McCrea MA, Nelson LD.) J Neurotrauma. 2025 Aug;42(15-16):1404-1415 PMID: 40200896 PMCID: PMC12431677 SCOPUS ID: 2-s2.0-105003876398 04/09/2025
-
(Nelson LD, Wilson L, Albrecht JS, Arciniegas DB, Barthélemy EJ, Fontaine SN, Gardner RC, Juengst SB, Pappadis MR, Ponsford J, Thomas DG, Yeates KO, Dams-O'Connor K, Manley GT, Maas AIR, McCrea MA, Awwad HO, Doperalski A, Umoh N.) J Neurotrauma. 2025 Jul;42(13-14):1023-1037 PMID: 40464097 PMCID: PMC12270537 SCOPUS ID: 2-s2.0-105009630976 06/04/2025
-
(Manley GT, Dams-O'Connor K, Alosco ML, Awwad HO, Bazarian JJ, Bragge P, Corrigan JD, Doperalski A, Ferguson AR, Mac Donald CL, Menon DK, McNett MM, van der Naalt J, Nelson LD, Pisică D, Silverberg ND, Umoh N, Wilson L, Yuh EL, Zetterberg H, Maas AIR, McCrea MA, NIH-NINDS TBI Classification and Nomenclature Initiative.) Lancet Neurol. 2025 Jun;24(6):512-523 PMID: 40409315 SCOPUS ID: 2-s2.0-105005509105 05/24/2025
-
(Arenth PM, Banos J, Bechtold K, Beck K, Biscardi M, Bodien Y, Botticello A, Brett B, Brodovsky J, Brostow D, Caccese JB, Callender L, Carrick F, Cook L, Cotner BA, Daugherty J, Dennison A, Diaz-Arrastia R, Douglas J, Eagle S, Edwards J, Evanson NK, Ferrington J, Finn JA, DeSalme EF, Fonda J, Fortier CB, Goldberg G, Graham J, Haag L, Hall C, Hanks R, Hansen C, Harris MB, Haun JN, Herrold A, Hicks SD, Hintze T, Hoffberg A, Hoffman J, Hoisington A, Holcomb EM, Howell DR, Jaffee M, Jaramillo CA, Kemp AM, Kennedy R, Kozlowski A, Kureshi N, Macgregor A, Martindale SL, Meulenbroek PA, Miles S, Mollayeva T, Nagele DA, Nakase-Richardson R, Nelson LD, Novack T, Novakovic-Agopian T, O'Brien K, O'Rourke JJJF, Orbo M, Parrott D, Perrin PB, Pogoda TK, Polzer E, Rabinowitz A, Reis D, Renshaw P, Ryan JL, Saadi A, Scholten J, Servatius R, Shafer ME, Shura R, Silva MA, AnnSisto S, Smith N, Sox-Harris A, Starosta AJ, Swan AA, Swank C, Theadom A, Tlustos S, Treble-Barna A, Turkstra LS, Valera E, Vaughan C, Weil Z, Weppner J, Whiteneck GG, Wortzel H, Yue JK.) Journal of Head Trauma Rehabilitation. 1 September 2025;40(5):393 PMID: 39969097 SCOPUS ID: 2-s2.0-105015561200 09/01/2025
-
(Nelson LD, Gray S, Barry CO, Young SA, deRoon-Cassini TA, Hedayat H, Kobayashi Y, McCrea MA, Tobes N, Trevino CM, Williams KS.) Mayo Clinic Proceedings Innovations Quality and Outcomes. August 2025;9(4) SCOPUS ID: 2-s2.0-105007887754 08/01/2025
-
(Manley GT, Dams-O'Connor K, Alosco ML, Awwad HO, Bazarian JJ, Bragge P, Corrigan JD, Doperalski A, Ferguson AR, Mac Donald CL, Menon DK, McNett MM, van der Naalt J, Nelson LD, Pisică D, Silverberg ND, Umoh N, Wilson L, Yuh EL, Zetterberg H, Maas AIR, McCrea MA.) Lancet Neurology. June 2025;24(6):512-523 SCOPUS ID: 2-s2.0-105005509105 06/01/2025
-
(Eagle SR, Barber J, Temkin N, McCrea MA, Giacino JT, Okonkwo DO, Madhok D, Yue JK, Zerbato JM, Manley GT, Nelson LD, TRACK-TBI Investigators.) Front Neurol. 2025;16:1558204 PMID: 40242629 PMCID: PMC12002085 04/17/2025

