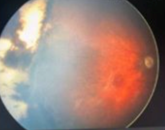
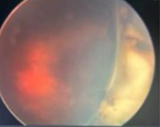
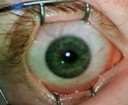
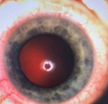
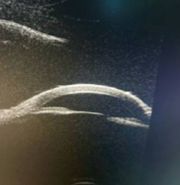
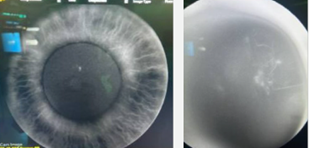
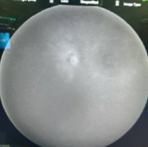
Case Study 25: 16-year-old non-verbal male with history of retinopathy of prematurity
Original Authors: Gao Zangzee Yang, Aparna Ramasubramanian, MD, Heather Stiff, MD
Medical College of Wisconsin Ophthalmology and Visual Sciences Case Studies
-
Ophthalmic Case Study 1
Acute right eye pain -
Ophthalmic Case Study 2
Red, itchy eyes -
Ophthalmic Case Study 3
Acute left eye pain and blurry vision -
Ophthalmic Case Study 4
Left eye pain and fuzzy vision 2 days after eye surgery -
Ophthalmic Case Study 5
Girl rubbing her R eye after trauma -
Ophthalmic Case Study 6
Red eye and pain on the left -
Ophthalmic Case Study 7
Vision loss L eye -
Ophthalmic Case Study 8
Crossed eyes -
Ophthalmic Case Study 9
White pupils -
Ophthalmic Case Study 10
Blurry vision in the left eye for 2 weeks -
Ophthalmic Case Study 11
Acute pain and burning in L eye -
Ophthalmic Case Study 12
Blurry vision in both eyes and headaches -
Ophthalmic Case Study 13
"Cannot see well" from left eye -
Ophthalmic Case Study 14
Blurry vision in both eyes -
Ophthalmic Case Study 15
Eye irritation and dryness -
Ophthalmic Case Study 16
2 brief episodes of vision loss in the R eye -
Ophthalmic Case Study 17
Routine eye exam -
Ophthalmic Case Study 18
8-year-old boy, difficulty seeing whiteboard -
Ophthalmic Case Study 19
8-year-old girl, wandering eye and double vision. -
Ophthalmic Case Study 20
Red eyelid lesion in an infant -
Ophthalmic Case Study 21
Female patient with optic nerve abnormality -
Ophthalmic Case Study 22
Left eye pain, tearing, redness, and photophobia -
Ophthalmic Case Study 23
1-year-old male presents with intermittent eye misalignment -
Ophthalmic Case Study 24
Left eye pain, redness, and blurry vision -
Ophthalmic Case Study 25
16-year-old non-verbal male with history of retinopathy -
Ophthalmic Case Study 26
69-year-old female presents for decreased visual acuity